Nicotine
 | |||
| |||
| Clinical data | |||
|---|---|---|---|
| Trade names | Nicorette, others | ||
| AHFS/Drugs.com | Monograph | ||
| Pregnancy category |
| ||
| Dependence liability | Physical: Low–moderate[1] Psychological: High[2][3] | ||
| Addiction liability | Very high in susceptible individuals[4] | ||
| Routes of administration | Inhalation; insufflation; oral – buccal, sublingual, and ingestion; transdermal; rectal | ||
| Drug class | Stimulant; Nootropic; Euphoriant | ||
| ATC code | |||
| Legal status | |||
| Legal status |
| ||
| Pharmacokinetic data | |||
| Protein binding | <5% | ||
| Metabolism | Primarily hepatic: CYP2A6, CYP2B6, FMO3, others | ||
| Metabolites | Cotinine | ||
| Elimination half-life | 1–2 hours; 20 hours active metabolite | ||
| Excretion | Renal, urine pH-dependent;[8] 10–20% (gum), 30% (inhaled); 10–30% (intranasal) | ||
| Identifiers | |||
| |||
| CAS Number | |||
| PubChem CID | |||
| IUPHAR/BPS | |||
| DrugBank | |||
| ChemSpider | |||
| UNII | |||
| KEGG | |||
| ChEBI | |||
| ChEMBL | |||
| PDB ligand | |||
| CompTox Dashboard (EPA) | |||
| ECHA InfoCard | 100.000.177 | ||
| Chemical and physical data | |||
| Formula | C10H14N2 | ||
| Molar mass | 162.236 g·mol−1 | ||
| 3D model (JSmol) | |||
| Chirality | Chiral | ||
| Density | 1.01 g/cm3 | ||
| Melting point | −79 °C (−110 °F) | ||
| Boiling point | 247 °C (477 °F) | ||
| |||
| |||
| Part of a series on |
| Tobacco |
|---|
 |
Nicotine is a naturally produced alkaloid in the nightshade family of plants (most predominantly in tobacco and Duboisia hopwoodii)[9] and is widely used recreationally as a stimulant and anxiolytic. As a pharmaceutical drug, it is used for smoking cessation to relieve withdrawal symptoms.[10][7][11][12] Nicotine acts as a receptor agonist at most nicotinic acetylcholine receptors (nAChRs),[13][14][15] except at two nicotinic receptor subunits (nAChRα9 and nAChRα10) where it acts as a receptor antagonist.[13]
Nicotine constitutes approximately 0.6–3.0% of the dry weight of tobacco.[16] Nicotine is also present at ppb concentrations in edible plants in the family Solanaceae, including potatoes, tomatoes, and eggplants,[17] though sources disagree on whether this has any biological significance to human consumers.[17] It functions as an antiherbivore toxin; consequently, nicotine was widely used as an insecticide in the past,[18][19] and neonicotinoids (structurally similar to nicotine), such as imidacloprid, are some of the most effective and widely used insecticides.
Nicotine is highly addictive.[20][21][22] Slow-release forms (gums and patches, when used correctly) can be less addictive and help in quitting.[23][24][25][26] Animal research suggests that monoamine oxidase inhibitors present in tobacco smoke may enhance nicotine's addictive properties.[27][28] An average cigarette yields about 2 mg of absorbed nicotine.[29] The estimated lower dose limit for fatal outcomes is 500–1,000 mg of ingested nicotine for an adult (6.5–13 mg/kg).[27][29] Nicotine addiction involves drug-reinforced behavior, compulsive use, and relapse following abstinence.[30] Nicotine dependence involves tolerance, sensitization,[31] physical dependence, psychological dependence,[32] and can cause distress.[33][34] Nicotine withdrawal symptoms include depressed mood, stress, anxiety, irritability, difficulty concentrating, and sleep disturbances.[2] Mild nicotine withdrawal symptoms are measurable in unrestricted smokers, who experience normal moods only as their blood nicotine levels peak, with each cigarette.[35] On quitting, withdrawal symptoms worsen sharply, then gradually improve to a normal state.[35]
Nicotine use as a tool for quitting smoking has a good safety history.[36] Animal studies suggest that nicotine may adversely affect cognitive development in adolescence, but the relevance of these findings to human brain development is disputed.[37][27] At low amounts, it has a mild analgesic effect.[38] According to the International Agency for Research on Cancer, "nicotine is not generally considered to be a carcinogen".[39][40] The Surgeon General of the United States indicates that evidence is inadequate to infer the presence or absence of a causal relationship between exposure to nicotine and risk for cancer.[41] Nicotine has been shown to produce birth defects in humans and is considered a teratogen.[42][43] The median lethal dose of nicotine in humans is unknown.[44] High doses are known to cause nicotine poisoning, organ failure, and death through paralysis of respiratory muscles,[41][45] though serious or fatal overdoses are rare.[46]
Uses
[edit]Medical
[edit]
The primary therapeutic use of nicotine is treating nicotine dependence to eliminate smoking and the damage it does to health. Controlled levels of nicotine are given to patients through gums, dermal patches, lozenges, inhalers, or nasal sprays to wean them off their dependence. A 2018 Cochrane Collaboration review found high-quality evidence that all current forms of nicotine replacement therapy (gum, patch, lozenges, inhaler, and nasal spray) increase the chances of successfully quitting smoking by 50–60%, regardless of setting.[47]
Combining nicotine patch use with a faster acting nicotine replacement, like gum or spray, improves the odds of treatment success.[48]
In contrast to recreational nicotine products, which have been designed to maximize the likelihood of addiction, nicotine replacement products (NRTs) are designed to minimize addictiveness.[41]: 112 The more quickly a dose of nicotine is delivered and absorbed, the higher the addiction risk.[33]
Investigative
Nicotine is being researched in clinical trials for possible benefit in treating Parkinson's disease, dementia, attention deficit hyperactivity disorder (ADHD), and depression.[49]
Nicotine may partly attenuate sensory gating and attentional deficits associated with schizophrenia. Short-term use of transdermal nicotine was found to improve subjects’ reaction time and alertness in given tasks. Nicotine was not found to improve negative, positive, or other cognitive symptoms of schizophrenia.[50]
Pesticide
[edit]Nicotine has been used as an insecticide since at least 1690, in the form of tobacco extracts or as pure nicotine sulphate[19][51][52] (although other components of tobacco also seem to have pesticide effects).[53] It acts on the nicotinic acetylcholine receptor, and gave the receptor its name. Nicotine is in IRAC group 4B. Nicotine insecticides have been banned in the US since 2014,[54] including use on organic crops,[55] and caution is recommended for small gardeners.[56] Nicotine pesticides have been banned in the EU since 2009.[57] Foods are imported from countries in which nicotine pesticides are allowed, such as China, but foods may not exceed maximum nicotine levels.[57][58] Neonicotinoids, such as imidacloprid, which are derived from and structurally similar to nicotine, are widely used as agricultural and veterinary pesticides as of 2016.[59][51]
Performance
[edit]Nicotine-containing products are sometimes used for the performance-enhancing effects of nicotine on cognition.[60] A 2010 meta-analysis of 41 double-blind, placebo-controlled studies concluded that nicotine or smoking had significant positive effects on aspects of fine motor abilities, alerting and orienting attention, and episodic and working memory.[61] A 2015 review noted that stimulation of the α4β2 nicotinic receptor is responsible for certain improvements in attentional performance;[62] among the nicotinic receptor subtypes, nicotine has the highest binding affinity at the α4β2 receptor (ki=1 nM), which is also the biological target that mediates nicotine's addictive properties.[63] Nicotine has potential beneficial effects, but it also has paradoxical effects, which may be due to the inverted U-shape of the dose-response curve or pharmacokinetic features.[64]
Recreational
[edit]Nicotine is used as a recreational drug.[65] It is widely used, highly addictive and hard to discontinue.[22] Nicotine is often used compulsively,[66] and dependence can develop within days.[66][67] Recreational drug users commonly use nicotine for its mood-altering effects.[33] Recreational nicotine products include chewing tobacco, cigars,[68] cigarettes,[68] e-cigarettes,[69] snuff, pipe tobacco,[68] snus, and nicotine pouches.
Alcohol infused with nicotine is called nicotini.[70]
Contraindications
[edit]Nicotine use for tobacco cessation has few contraindications.[71]
It is not known whether nicotine replacement therapy is effective for smoking cessation in adolescents, as of 2014.[72] It is therefore not recommended to adolescents.[73] It is not safe to use nicotine during pregnancy or breastfeeding, although it is safer than smoking. The desirability of NRT use in pregnancy is therefore debated.[74][75][76]
Randomized trials and observational studies of nicotine replacement therapy in cardiovascular patients show no increase in adverse cardiovascular events compared to those treated with placebo.[77] Using nicotine products during cancer treatment may be contraindicated, as nicotine may promote tumour growth, but temporary use of NRTs to quit smoking may be advised for harm reduction.[78]
Nicotine gum is contraindicated in individuals with temporomandibular joint disease.[79] People with chronic nasal disorders and severe reactive airway disease require additional precautions when using nicotine nasal sprays.[73] Nicotine in any form is contraindicated in individuals with a known hypersensitivity to nicotine.[79][73]
Adverse effects
[edit]
Nicotine is classified as a poison,[81][82] and it is "extremely hazardous".[83] However, at doses typically used by consumers, it presents little if any hazard to the user.[84][85][86] A 2018 Cochrane Collaboration review lists nine main adverse events related to nicotine replacement therapy: headache, dizziness, lightheadedness, nausea, vomiting, gastrointestinal symptoms, insomnia, abnormal dreams, non-ischemic palpitations and chest pain, skin reactions, oral/nasal reactions, and hiccups.[87] Many of these were also common in the placebo group without nicotine.[87] Palpitations and chest pain were deemed "rare" and there was no evidence of an increased number of serious cardiac problems compared to the placebo group, even in people with established cardiac disease.[47] The common side effects from nicotine exposure are listed in the table below. Serious adverse events due to the use of nicotine replacement therapy are extremely rare.[47] At low amounts, it has a mild analgesic effect.[38] However, at sufficiently high doses, nicotine may result in nausea, vomiting, diarrhea, salivation, bradycardia, and possibly seizures, hypoventilation, and death.[88]
| Route of administration | Dosage form | Associated side effects of nicotine | Sources |
|---|---|---|---|
| Buccal | Nicotine gum | Indigestion, nausea, hiccups, traumatic injury to oral mucosa or teeth, irritation or tingling of the mouth and throat, oral mucosal ulceration, jaw-muscle ache, burping, gum sticking to teeth, unpleasant taste, dizziness, lightheadedness, headache, and insomnia. | [47][79] |
| Lozenge | Nausea, dyspepsia, flatulence, headache, upper respiratory tract infections, irritation (i.e., a burning sensation), hiccups, sore throat, coughing, dry lips, and oral mucosal ulceration. | [47][79] | |
| Transdermal | Transdermal patch |
Application site reactions (i.e., pruritus, burning, or erythema), diarrhea, dyspepsia, abdominal pain, dry mouth, nausea, dizziness, nervousness or restlessness, headache, vivid dreams or other sleep disturbances, and irritability. | [47][79][89] |
| Intranasal | Nasal spray | Runny nose, nasopharyngeal and ocular irritation, watery eyes, sneezing, and coughing. | [47][79][90] |
| Oral inhalation | Inhaler | Dyspepsia, oropharyngeal irritation (e.g., coughing, irritation of the mouth and throat), rhinitis, and headache. | [47][79][91] |
| All (nonspecific) | Peripheral vasoconstriction, tachycardia (i.e., fast heart rate), elevated blood pressure, increased alertness and cognitive performance. | [79][90] | |
Sleep
[edit]Nicotine reduces the amount of rapid eye movement (REM) sleep, slow-wave sleep (SWS), and total sleep time in healthy nonsmokers given nicotine via a transdermal patch, and the reduction is dose-dependent.[92] Acute nicotine intoxication has been found to significantly reduce total sleep time and increase REM latency, sleep onset latency, and non-rapid eye movement (NREM) stage 2 sleep time.[92][93] Depressive non-smokers experience mood and sleep improvements under nicotine administration; however, subsequent nicotine withdrawal has a negative effect on both mood and sleep.[94]
Cardiovascular system
[edit]Nicotine exerts several significant effects on the cardiovascular system. Primarily, it stimulates the sympathetic nervous system, leading to the release of catecholamines. This activation results in an increase in heart rate and blood pressure, as well as enhanced myocardial contractility, which raises the workload on the heart. Additionally, nicotine causes systemic vasoconstriction, including constriction of coronary arteries, which can reduce blood flow to the heart. Long-term exposure to nicotine may impair endothelial function, potentially contributing to atherosclerosis. Furthermore, nicotine has been associated with the development of cardiac arrhythmias, particularly in individuals who already have underlying heart disease.[95]
The effects of nicotine can be differentiated between short-term and long-term use. Short-term nicotine use, such as that associated with nicotine replacement therapy (NRT) for smoking cessation, appears to pose little cardiovascular risk, even for patients with known cardiovascular conditions. In contrast, longer-term nicotine use may not accelerate atherosclerosis but could contribute to acute cardiovascular events in those with pre-existing cardiovascular disease. Many severe cardiovascular effects traditionally associated with smoking may not be solely attributable to nicotine itself. Cigarette smoke contains numerous other potentially cardiotoxic substances, including carbon monoxide and oxidant gases.[95]
A 2016 review of the cardiovascular toxicity of nicotine concluded, "Based on current knowledge, we believe that the cardiovascular risks of nicotine from e-cigarette use in people without cardiovascular disease are quite low. We have concerns that nicotine from e-cigarettes could pose some risk for users with cardiovascular disease."[95]
A 2018 Cochrane review found that, in rare cases, nicotine replacement therapy can cause non-ischemic chest pain (i.e., chest pain that is unrelated to a heart attack) and heart palpitations, but does not increase the incidence of serious cardiac adverse events (i.e., myocardial infarction, stroke, and cardiac death) relative to controls.[47]
Blood pressure
[edit]In the short term, nicotine causes a transient increase in blood pressure. Long term, epidemiological studies generally show increased blood pressure and hypertension among nicotine users.[95]
Reinforcement disorders
[edit]ΔFosB accumulation from excessive drug use
Top: this depicts the initial effects of high dose exposure to an addictive drug on gene expression in the nucleus accumbens for various Fos family proteins (i.e., c-Fos, FosB, ΔFosB, Fra1, and Fra2).
Bottom: this illustrates the progressive increase in ΔFosB expression in the nucleus accumbens following repeated twice daily drug binges, where these phosphorylated (35–37 kilodalton) ΔFosB isoforms persist in the D1-type medium spiny neurons of the nucleus accumbens for up to 2 months.[96][97] |
Nicotine is highly addictive but paradoxically has quite weak reinforcing property compared to other drugs of abuse in various animals.[21][22][98][99] Its addictiveness depends on how it is administered and also depends upon form in which nicotine is used.[25] Animal research suggests that monoamine oxidase inhibitors, acetaldehyde[99][100] and other constituents in tobacco smoke may enhance its addictiveness.[27][28] Nicotine dependence involves aspects of both psychological dependence and physical dependence, since discontinuation of extended use has been shown to produce both affective (e.g., anxiety, irritability, craving, anhedonia) and somatic (mild motor dysfunctions such as tremor) withdrawal symptoms.[2] Withdrawal symptoms peak in one to three days[101] and can persist for several weeks.[102] Even though other drugs of dependence can have withdrawal states lasting 6 months or longer, this does not appear to occur with cigarette withdrawal.[103]
Normal between-cigarettes discontinuation, in unrestricted smokers, causes mild but measurable nicotine withdrawal symptoms.[35] These include mildly worse mood, stress, anxiety, cognition, and sleep, all of which briefly return to normal with the next cigarette.[35] Smokers have a worse mood than they typically would have if they were not nicotine-dependent; they experience normal moods only immediately after smoking.[35] Nicotine dependence is associated with poor sleep quality and shorter sleep duration among smokers.[104][105]
In dependent smokers, withdrawal causes impairments in memory and attention, and smoking during withdrawal returns these cognitive abilities to pre-withdrawal levels.[106] The temporarily increased cognitive levels of smokers after inhaling smoke are offset by periods of cognitive decline during nicotine withdrawal.[35] Therefore, the overall daily cognitive levels of smokers and non-smokers are roughly similar.[35]
Nicotine activates the mesolimbic pathway and induces long-term ΔFosB expression (i.e., produces phosphorylated ΔFosB isoforms) in the nucleus accumbens when inhaled or injected frequently or at high doses, but not necessarily when ingested.[107][108][109] Consequently, high daily exposure (possibly excluding oral route) to nicotine can cause ΔFosB overexpression in the nucleus accumbens, resulting in nicotine addiction.[107][108]
Cancer
[edit]Contrary to popular belief, nicotine itself does not cause cancer in humans,[40][110] although it is unclear whether it functions as a tumor promoter as of 2012[update].[111] A 2018 report by the US National Academies of Sciences, Engineering, and Medicine concludes, "[w]hile it is biologically plausible that nicotine can act as a tumor promoter, the existing body of evidence indicates this is unlikely to translate into increased risk of human cancer."[112]
Although nicotine is classified as a non-carcinogenic substance, it can still promote tumor growth and metastasis. It induces several processes that contribute to cancer progression, including cell cycle progression, epithelial-to-mesenchymal transition, migration, invasion, angiogenesis, and evasion of apoptosis.[113] These effects are primarily mediated through nicotinic acetylcholine receptors (nAChRs), particularly the α7 subtype, and to a lesser extent, β-adrenergic receptors (β-ARs). Activation of these receptors triggers several signaling cascades crucial in cancer biology, notably the MAPK/ERK pathway, PI3K/AKT pathway, and JAK-STAT signaling.[113]
Nicotine promotes lung cancer development by enhancing proliferation, angiogenesis, migration, invasion, and epithelial–mesenchymal transition (EMT) via nAChRs, which are present in lung cancer cells.[114] Additionally, nicotine-induced EMT contributes to drug resistance in cancer cells.[115]
Nicotine in tobacco can form carcinogenic tobacco-specific nitrosamines through a nitrosation reaction. This occurs mostly in the curing and processing of tobacco. However, nicotine in the mouth and stomach can react to form N-nitrosonornicotine,[116] a known type 1 carcinogen,[117] suggesting that consumption of non-tobacco forms of nicotine may still play a role in carcinogenesis.[118]
Genotoxicity
[edit]Nicotine causes DNA damage in several types of human cells as judged by assays for genotoxicity such as the comet assay, cytokinesis-block micronucleus test and chromosome aberrations test. In humans, this damage can happen in primary parotid gland cells,[119] lymphocytes,[120] and respiratory tract cells.[121]
Pregnancy and breastfeeding
[edit]Nicotine has been shown to produce birth defects in some animal species, but not others;[43] consequently, it is considered to be a possible teratogen in humans.[43] In animal studies that resulted in birth defects, researchers found that nicotine negatively affects fetal brain development and pregnancy outcomes;[43][41] the negative effects on early brain development are associated with abnormalities in brain metabolism and neurotransmitter system function.[122] Nicotine crosses the placenta and is found in the breast milk of mothers who smoke as well as mothers who inhale passive smoke.[123]
Nicotine exposure in utero is responsible for several complications of pregnancy and birth: pregnant women who smoke are at greater risk for both miscarriage and stillbirth and infants exposed to nicotine in utero tend to have lower birth weights.[124] A McMaster University research group observed in 2010 that rats exposed to nicotine in the womb (via parenteral infusion) later in life had conditions including type 2 diabetes, obesity, hypertension, neurobehavioral defects, respiratory dysfunction, and infertility.[125]
Overdose
[edit]It is unlikely that a person would overdose on nicotine through smoking alone. The US Food and Drug Administration (FDA) stated in 2013 that there are no significant safety concerns associated with the use of more than one form of over-the-counter (OTC) nicotine replacement therapy at the same time, or using OTC NRT at the same time as another nicotine-containing product, like cigarettes.[126] The median lethal dose of nicotine in humans is unknown.[44][29] Nevertheless, nicotine has a relatively high toxicity in comparison to many other alkaloids such as caffeine, which has an LD50 of 127 mg/kg when administered to mice.[127] At sufficiently high doses, it is associated with nicotine poisoning,[41] which, while common in children (in whom poisonous and lethal levels occur at lower doses per kilogram of body weight[38]) rarely results in significant morbidity or death.[43] The estimated lower dose limit for fatal outcomes is 500–1,000 mg of ingested nicotine for an adult (6.5–13 mg/kg).[27][29]
The initial symptoms of a nicotine overdose typically include nausea, vomiting, diarrhea, hypersalivation, abdominal pain, tachycardia (rapid heart rate), hypertension (high blood pressure), tachypnea (rapid breathing), headache, dizziness, pallor (pale skin), auditory or visual disturbances, and perspiration, followed shortly after by marked bradycardia (slow heart rate), bradypnea (slow breathing), and hypotension (low blood pressure).[43] An increased respiratory rate (i.e., tachypnea) is one of the primary signs of nicotine poisoning.[43] At sufficiently high doses, somnolence (sleepiness or drowsiness), confusion, syncope (loss of consciousness from fainting), shortness of breath, marked weakness, seizures, and coma may occur.[8][43] Lethal nicotine poisoning rapidly produces seizures, and death – which may occur within minutes – is believed to be due to respiratory paralysis.[43]
Toxicity
[edit]Today nicotine is less commonly used in agricultural insecticides, which was a main source of poisoning. More recent cases of poisoning typically appear to be in the form of Green Tobacco Sickness (GTS),[43] accidental ingestion of tobacco or tobacco products, or ingestion of nicotine-containing plants.[128][129][130] People who harvest or cultivate tobacco may experience GTS, a type of nicotine poisoning caused by dermal exposure to wet tobacco leaves. This occurs most commonly in young, inexperienced tobacco harvesters who do not consume tobacco.[128][131] People can be exposed to nicotine in the workplace by breathing it in, skin absorption, swallowing it, or eye contact. The Occupational Safety and Health Administration (OSHA) has set the legal limit (permissible exposure limit) for nicotine exposure in the workplace as 0.5 mg/m3 skin exposure over an 8-hour workday. The US National Institute for Occupational Safety and Health (NIOSH) has set a recommended exposure limit (REL) of 0.5 mg/m3 skin exposure over an 8-hour workday. At environmental levels of 5 mg/m3, nicotine is immediately dangerous to life and health.[132]
Drug interactions
[edit]Pharmacodynamic
[edit]- Potential interaction with sympathomimetic drugs (adrenergic agonists) and sympatholytic drugs (alpha-blockers and beta-blockers).[79]
Pharmacokinetic
[edit]Nicotine and cigarette smoke both induce the expression of liver enzymes (e.g., certain cytochrome P450 proteins) which metabolize drugs, leading to the potential for alterations in drug metabolism.[79]
- Smoking cessation may decrease the metabolism of acetaminophen, beta-blockers, caffeine, oxazepam, pentazocine, propoxyphene, theophylline, and tricyclic antidepressants, leading to higher plasma concentrations of these drugs.[79]
- Possible alteration of nicotine absorption through the skin from the transdermal nicotine patch by drugs that cause vasodilation or vasoconstriction.[79]
- Possible alteration of nicotine absorption through the nasal cavity from the nicotine nasal spray by nasal vasoconstrictors (e.g., xylometazoline).[79]
- Possible alteration of nicotine absorption through oral mucosa from nicotine gum and lozenges by food and drink that modify salivary pH.[79]
Pharmacology
[edit]Pharmacodynamics
[edit]Nicotine acts as a receptor agonist at most nicotinic acetylcholine receptors (nAChRs),[13][14] except at two nicotinic receptor subunits (nAChRα9 and nAChRα10) where it acts as a receptor antagonist.[13] Such antagonism results in mild analgesia.
Central nervous system
[edit]
By binding to nicotinic acetylcholine receptors in the brain, nicotine elicits its psychoactive effects and increases the levels of several neurotransmitters in various brain structures – acting as a sort of "volume control".[133][134] Nicotine has a higher affinity for nicotinic receptors in the brain than those in skeletal muscle, though at toxic doses it can induce contractions and respiratory paralysis.[135] Nicotine's selectivity is thought to be due to a particular amino acid difference on these receptor subtypes.[136] Nicotine is unusual in comparison to most drugs, as its profile changes from stimulant to sedative with increasing dosages, a phenomenon known as "Nesbitt's paradox" after the doctor who first described it in 1969.[137][138] At very high doses it dampens neuronal activity.[139] Nicotine induces both behavioral stimulation and anxiety in animals.[8] Research into nicotine's most predominant metabolite, cotinine, suggests that some of nicotine's psychoactive effects are mediated by cotinine.[140]
Nicotine activates nicotinic receptors (particularly α4β2 nicotinic receptors, but also α5 nAChRs) on neurons that innervate the ventral tegmental area and within the mesolimbic pathway where it appears to cause the release of dopamine.[141][142] This nicotine-induced dopamine release occurs at least partially through activation of the cholinergic–dopaminergic reward link in the ventral tegmental area.[142][143] Nicotine can modulate the firing rate of the ventral tegmental area neurons.[143] These actions are largely responsible for the strongly reinforcing effects of nicotine, which often occur in the absence of euphoria;[141] however, mild euphoria from nicotine use can occur in some individuals.[141] Chronic nicotine use inhibits class I and II histone deacetylases in the striatum, where this effect plays a role in nicotine addiction.[144][145]
Sympathetic nervous system
[edit]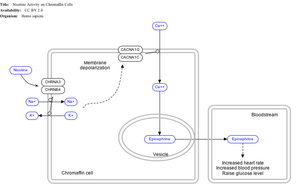
Nicotine also activates the sympathetic nervous system,[146] acting via splanchnic nerves to the adrenal medulla, stimulating the release of epinephrine. Acetylcholine released by preganglionic sympathetic fibers of these nerves acts on nicotinic acetylcholine receptors, causing the release of epinephrine (and norepinephrine) into the bloodstream.
Adrenal medulla
[edit]By binding to ganglion type nicotinic receptors in the adrenal medulla, nicotine increases flow of adrenaline (epinephrine), a stimulating hormone and neurotransmitter. By binding to the receptors, it causes cell depolarization and an influx of calcium through voltage-gated calcium channels. Calcium triggers the exocytosis of chromaffin granules and thus the release of epinephrine (and norepinephrine) into the bloodstream. The release of epinephrine (adrenaline) causes an increase in heart rate, blood pressure and respiration, as well as higher blood glucose levels.[147]
Pharmacokinetics
[edit]
As nicotine enters the body, it is distributed quickly through the bloodstream and crosses the blood–brain barrier reaching the brain within 10–20 seconds after inhalation.[149] The elimination half-life of nicotine in the body is around two hours.[150][151] Nicotine is primarily excreted in urine and urinary concentrations vary depending upon urine flow rate and urine pH.[8]
The amount of nicotine absorbed by the body from smoking can depend on many factors, including the types of tobacco, whether the smoke is inhaled, and whether a filter is used. However, it has been found that the nicotine yield of individual products has only a small effect (4.4%) on the blood concentration of nicotine,[152] suggesting "the assumed health advantage of switching to lower-tar and lower-nicotine cigarettes may be largely offset by the tendency of smokers to compensate by increasing inhalation".
Nicotine has a half-life of 1–2 hours. Cotinine is an active metabolite of nicotine that remains in the blood with a half-life of 18–20 hours, making it easier to analyze.[153]
Nicotine is metabolized in the liver by cytochrome P450 enzymes (mostly CYP2A6, and also by CYP2B6) and FMO3, which selectively metabolizes (S)-nicotine. A major metabolite is cotinine. Other primary metabolites include nicotine N-oxide, nornicotine, nicotine isomethonium ion, 2-hydroxynicotine and nicotine glucuronide.[154] Under some conditions, other substances may be formed such as myosmine.[155][156]
Glucuronidation and oxidative metabolism of nicotine to cotinine are both inhibited by menthol, an additive to mentholated cigarettes, thus increasing the half-life of nicotine in vivo.[157]
Metabolism
[edit]Nicotine decreases hunger and as a consequence food consumption, alongside increasing energy expenditure.[158][159] The majority of research shows that nicotine reduces body weight, but some researchers have found that nicotine may result in weight gain under specific types of eating habits in animal models.[159] Nicotine effect on weight appears to result from nicotine's stimulation of α3β4 nAChR receptors located in the POMC neurons in the arcuate nucleus and subsequently the melanocortin system, especially the melanocortin-4 receptors on second-order neurons in the paraventricular nucleus of the hypothalamus, thus modulating feeding inhibition.[143][159] POMC neurons are a precursor of the melanocortin system, a critical regulator of body weight and peripheral tissue such as skin and hair.[159]
Chemistry
[edit]| NFPA 704 safety square | |
|---|---|
The fire diamond hazard sign for nicotine[160] |
Nicotine is a hygroscopic, colorless[83] to yellow-brown, oily liquid, that is readily soluble in alcohol, ether or light petroleum. It is miscible with water in its neutral amine base form between 60 °C and 210 °C. It is a dibasic nitrogenous base, having Kb1=1×10−6, Kb2=1×10−11.[161] It readily forms ammonium salts with acids that are usually solid and water-soluble. Its flash point is 95 °C and its auto-ignition temperature is 244 °C.[162] Nicotine is readily volatile (vapor pressure 5.5 Pa at 25 °C)[161] On exposure to ultraviolet light or various oxidizing agents, nicotine is converted to nicotine oxide, nicotinic acid (niacin, vitamin B3), and methylamine.[163]
Nicotine is chiral and hence optically active, having two enantiomeric forms. The naturally occurring form of nicotine is levorotatory with a specific rotation of [α]D=–166.4° ((−)-nicotine). The dextrorotatory form, (+)-nicotine is physiologically less active than (−)-nicotine. (−)-nicotine is more toxic than (+)-nicotine.[164] The salts of (−)-nicotine are usually dextrorotatory; this conversion between levorotatory and dextrorotatory upon protonation is common among alkaloids.[163] The hydrochloride and sulfate salts become optically inactive if heated in a closed vessel above 180 °C.[163] Anabasine is a structural isomer of nicotine, as both compounds have the molecular formula C10H14N2.
Nicotine that is found in natural tobacco is primarily (99%) the S-enantiomer.[165] Conversely, the most common chemistry synthetic methods for generating nicotine yields a product that is approximately equal proportions of the S- and R-enantiomers.[166] This suggests that tobacco-derived and synthetic nicotine can be determined by measuring the ratio of the two different enantiomers, although means exist for adjusting the relative levels of the enantiomers or performing a synthesis that only leads to the S-enantiomer. There is limited data on the relative physiological effects of these two enantiomers, especially in people. However, the studies to date indicate that (S)-nicotine is more potent than (R)-nicotine and (S)-nicotine causes stronger sensations or irritation than (R)-nicotine. Studies have not been adequate to determine the relative addictiveness of the two enantiomers in people.

Pod mod electronic cigarettes use nicotine in the form of a protonated nicotine, rather than free-base nicotine found in earlier generations.[167]
Preparation
[edit]The first laboratory preparation of nicotine (as its racemate) was described in 1904.[168]
 The starting material was an N-substituted pyrrole derivative, which was heated to convert it by a [1,5] sigmatropic shift to the isomer with a carbon bond between the pyrrole and pyridine rings, followed by methylation and selective reduction of the pyrrole ring using tin and hydrochloric acid.[168][169] Many other syntheses of nicotine, in both racemic and chiral forms have since been published.[170]
The starting material was an N-substituted pyrrole derivative, which was heated to convert it by a [1,5] sigmatropic shift to the isomer with a carbon bond between the pyrrole and pyridine rings, followed by methylation and selective reduction of the pyrrole ring using tin and hydrochloric acid.[168][169] Many other syntheses of nicotine, in both racemic and chiral forms have since been published.[170]
Biosynthesis
[edit]
The biosynthetic pathway of nicotine involves a coupling reaction between the two cyclic structures that comprise nicotine. Metabolic studies show that the pyridine ring of nicotine is derived from niacin (nicotinic acid) while the pyrrolidine is derived from N-methyl-Δ1-pyrrollidium cation.[171][172] Biosynthesis of the two component structures proceeds via two independent syntheses, the NAD pathway for niacin and the tropane pathway for N-methyl-Δ1-pyrrollidium cation.
The NAD pathway in the genus Nicotiana begins with the oxidation of aspartic acid into α-amino succinate by aspartate oxidase (AO). This is followed by a condensation with glyceraldehyde-3-phosphate and a cyclization catalyzed by quinolinate synthase (QS) to give quinolinic acid. Quinolinic acid then reacts with phosphoribosyl pyrophosphate catalyzed by quinolinic acid phosphoribosyl transferase (QPT) to form niacin mononucleotide (NaMN). The reaction now proceeds via the NAD salvage cycle to produce niacin via the conversion of nicotinamide by the enzyme nicotinamidase.[citation needed]
The N-methyl-Δ1-pyrrollidium cation used in the synthesis of nicotine is an intermediate in the synthesis of tropane-derived alkaloids. Biosynthesis begins with decarboxylation of ornithine by ornithine decarboxylase (ODC) to produce putrescine. Putrescine is then converted into N-methyl putrescine via methylation by SAM catalyzed by putrescine N-methyltransferase (PMT). N-methyl putrescine then undergoes deamination into 4-methylaminobutanal by the N-methyl putrescine oxidase (MPO) enzyme, 4-methylaminobutanal then spontaneously cyclize into N-methyl-Δ1-pyrrollidium cation.[citation needed]
The final step in the synthesis of nicotine is the coupling between N-methyl-Δ1-pyrrollidium cation and niacin. Although studies conclude some form of coupling between the two component structures, the definite process and mechanism remains undetermined. The current agreed theory involves the conversion of niacin into 2,5-dihydropyridine through 3,6-dihydronicotinic acid. The 2,5-dihydropyridine intermediate would then react with N-methyl-Δ1-pyrrollidium cation to form enantiomerically pure (−)-nicotine.[173]
Detection in body fluids
[edit]Nicotine can be quantified in blood, plasma, or urine to confirm a diagnosis of poisoning or to facilitate a medicolegal death investigation. Urinary or salivary cotinine concentrations are frequently measured for the purposes of pre-employment and health insurance medical screening programs. Careful interpretation of results is important, since passive exposure to cigarette smoke can result in significant accumulation of nicotine, followed by the appearance of its metabolites in various body fluids.[174][175] Nicotine use is not regulated in competitive sports programs.[176]
Methods for analysis of enantiomers
[edit]Methods for measuring the two enantiomers are straightforward and include normal-phase liquid chromatography,[165] liquid chromatography with a chiral column.[177] However, since methods can be used to alter the two enantiomers, it may not be possible to distinguish tobacco-derived from synthetic nicotine simply by measuring the levels of the two enantiomers. A new approach uses hydrogen and deuterium nuclear magnetic resonance to distinguish tobacco-derived and synthetic nicotine based on differences the substrates used in the natural synthetic pathway performed in the tobacco plant and the substrates most used in synthesis.[178] Another approach measures the carbon-14 content which also differs between natural and laboratory-based tobacco.[179] These methods remain to be fully evaluated and validated using a wide range of samples.
Natural occurrence
[edit]Nicotine is a secondary metabolite produced in a variety of plants in the family Solanaceae, most notably in tobacco Nicotiana tabacum, where it can be found at high concentrations of 0.5 to 7.5%.[180] Nicotine is also found in the leaves of other tobacco species, such as Nicotiana rustica (in amounts of 2–14%). Nicotine production is strongly induced in response to wounding as part of a jasmonate-dependent reaction.[181] Specialist insects on tobacco, such as the tobacco hornworm (Manduca sexta), have a number of adaptations to the detoxification and even adaptive re-purposing of nicotine.[182] Nicotine is also found at low concentrations in the nectar of tobacco plants, where it may promote outcrossing by affecting the behavior of hummingbird pollinators.[183]
Nicotine occurs in smaller amounts (varying from 2–7 μg/kg, or 20–70 millionths of a percent wet weight[17]) in other Solanaceaeous plants, including some crop species such as potatoes, tomatoes, eggplant, and peppers,[17][184] as well as non-crop species such as Duboisia hopwoodii.[161] The amounts of nicotine in tomatoes lowers substantially as the fruit ripens.[17] A 1999 report found "In some papers it is suggested that the contribution of dietary nicotine intake is significant when compared with exposure to ETS [environmental tobacco smoke] or by active smoking of small numbers of cigarettes. Others consider the dietary intake to be negligible unless inordinately large amounts of specific vegetables are consumed."[17] The amount of nicotine eaten per day is roughly around 1.4 and 2.25 μg/day at the 95th percentile.[17] These numbers may be low due to insufficient food intake data.[17] The concentrations of nicotine in vegetables are difficult to measure accurately, since they are very low (parts per billion range).[185] Pure nicotine tastes "terrible".[83]
History, society and culture
[edit]
Nicotine was originally isolated from the tobacco plant in 1828 by chemists Wilhelm Heinrich Posselt and Karl Ludwig Reimann from Germany, who believed it was a poison.[186][187] Its chemical empirical formula was described by Melsens in 1843,[188] its structure was discovered by Adolf Pinner and Richard Wolffenstein in 1893,[189][190][191][clarification needed] and it was first synthesized by Amé Pictet and A. Rotschy in 1904.[168][192]
Nicotine is named after the tobacco plant Nicotiana tabacum, which in turn is named after the French ambassador in Portugal, Jean Nicot de Villemain, who sent tobacco and seeds to Paris in 1560, presented to the French King,[193] and who promoted their medicinal use. Smoking was believed to protect against illness, particularly the plague.[193]
Tobacco was introduced to Europe in 1559, and by the late 17th century, it was used not only for smoking but also as an insecticide. After World War II, over 2,500 tons of nicotine insecticide were used worldwide, but by the 1980s the use of nicotine insecticide had declined below 200 tons. This was due to the availability of other insecticides that are cheaper and less harmful to mammals.[19]
The nicotine content of popular American-brand cigarettes has increased over time, and one study found that there was an average increase of 1.78% per year between the years of 1998 and 2005.[194]
Although methods of production of synthetic nicotine have existed for decades,[195] it was believed that the cost of making nicotine by laboratory synthesis was cost prohibitive compared to extracting nicotine from tobacco.[196] However, recently synthetic nicotine started to be found in different brands of e-cigarettes and oral pouches and marketed as "tobacco-free."[197]
The US FDA is tasked with reviewing tobacco products such as e-cigarettes and determining which can be authorized for sale. In response to the likelihood that FDA would not authorize many e-cigarettes to be marketed, e-cigarette companies began marketing products that they claimed to contain nicotine that were not made or derived from tobacco, but contained synthetic nicotine instead, and thus, would be outside FDA's tobacco regulatory authority.[198] Similarly, nicotine pouches that claimed to contain non-tobacco (synthetic) nicotine were also introduced. The cost of synthetic nicotine has decreased as the market for the product increased. In March 2022, the U.S. Congress passed a law (the Consolidated Appropriations Act, 2022) that expanded FDA's tobacco regulatory authority to include tobacco products containing nicotine from any source, thereby including products made with synthetic nicotine.
Legal status
[edit]In the United States, nicotine products and nicotine replacement therapy products like Nicotrol are only available to people 18 and above; proof of age is required; not for sale in vending machine or from any source where proof of age cannot be verified. As of 2019, the minimum age to purchase tobacco in the US is 21 at the federal level.[199]
In the European Union, the minimum age to purchase nicotine products is 18. However, there is no minimum age requirement to use tobacco or nicotine products.[200]
In the United Kingdom, the Tobacco and Related Products Regulations 2016 implemented the European directive 2014/40/EU, amended by Tobacco Products and Nicotine Inhaling Products (Amendment etc.) (EU Exit) Regulations 2019 and the Tobacco Products and Nicotine Inhaling Products (Amendment) (EU Exit) Regulations 2020. Additionally other regulations limit advertising, sale and display of tobacco products and other products containing nicotine for human consumption. The Sunak government proposed banning disposable vapes to limit their appeal and affordability for children and to reduce the amount of waste generated.
In media
[edit]| External image | |
|---|---|
In some anti-smoking literature, the harm that tobacco smoking and nicotine addiction does is personified as Nick O'Teen, represented as a humanoid with some aspect of a cigarette or cigarette butt about him or his clothes and hat.[201] Nick O'Teen was a villain that was created for the Health Education Council. The character was featured in three animated anti-smoking public service announcements in which he tries to get kids addicted to cigarettes before being foiled by the DC Comics character Superman.[201]
Nicotine was often compared to caffeine in advertisements in the 1980s by the tobacco industry, and later in the 2010s by the electronic cigarettes industry, in an effort to reduce the stigmatization and the public perception of the risks associated with nicotine use.[202]
Research
[edit]Central nervous system
[edit]While acute/initial nicotine intake causes activation of neuronal nicotine receptors, chronic low doses of nicotine use leads to desensitization of those receptors (due to the development of tolerance) and results in an antidepressant effect, with early research showing low dose nicotine patches could be an effective treatment of major depressive disorder in non-smokers.[203]
Though tobacco smoking is associated with an increased risk of Alzheimer's disease,[204] there is evidence that nicotine itself has the potential to prevent and treat Alzheimer's disease.[205]
Smoking is associated with a decreased risk of Parkinson's disease; however, it is unknown whether this is due to people with healthier brain dopaminergic reward centers (the area of the brain affected by Parkinson's) being more likely to enjoy smoking and thus pick up the habit, nicotine directly acting as a neuroprotective agent, or other compounds in cigarette smoke acting as neuroprotective agents.[206]
Immune system
[edit]Immune cells of both the innate immune system and adaptive immune systems frequently express the α2, α5, α6, α7, α9, and α10 subunits of nicotinic acetylcholine receptors.[207] Evidence suggests that nicotinic receptors which contain these subunits are involved in the regulation of immune function.[207]
Optopharmacology
[edit]A photoactivatable form of nicotine, which releases nicotine when exposed to ultraviolet light with certain conditions, has been developed for studying nicotinic acetylcholine receptors in brain tissue.[208]
Oral health
[edit]Several in vitro studies have investigated the potential effects of nicotine on a range of oral cells. A recent systematic review concluded that nicotine was unlikely to be cytotoxic to oral cells in vitro in most physiological conditions but further research is needed.[209] Understanding the potential role of nicotine in oral health has become increasingly important given the recent introduction of novel nicotine products and their potential role in helping smokers quit.[210]
See also
[edit]References
[edit]- ^ McLaughlin I, Dani JA, De Biasi M (2015). "Nicotine Withdrawal". The Neuropharmacology of Nicotine Dependence. Current Topics in Behavioral Neurosciences. Vol. 24. pp. 99–123. doi:10.1007/978-3-319-13482-6_4. ISBN 978-3-319-13481-9. PMC 4542051. PMID 25638335.
- ^ a b c D'Souza MS, Markou A (July 2011). "Neuronal mechanisms underlying development of nicotine dependence: implications for novel smoking-cessation treatments". Addiction Science & Clinical Practice. 6 (1): 4–16. PMC 3188825. PMID 22003417.
Withdrawal symptoms upon cessation of nicotine intake: Chronic nicotine use induces neuroadaptations in the brain's reward system that result in the development of nicotine dependence. Thus, nicotine-dependent smokers must continue nicotine intake to avoid distressing somatic and affective withdrawal symptoms. Newly abstinent smokers experience symptoms such as depressed mood, anxiety, irritability, difficulty concentrating, craving, bradycardia, insomnia, gastrointestinal discomfort, and weight gain (Shiffman and Jarvik, 1976; Hughes et al., 1991). Experimental animals, such as rats and mice, exhibit a nicotine withdrawal syndrome that, like the human syndrome, includes both somatic signs and a negative affective state (Watkins et al., 2000; Malin et al., 2006). The somatic signs of nicotine withdrawal include rearing, jumping, shakes, abdominal constrictions, chewing, scratching, and facial tremors. The negative affective state of nicotine withdrawal is characterized by decreased responsiveness to previously rewarding stimuli, a state called anhedonia.
- ^ Cosci F, Pistelli F, Lazzarini N, Carrozzi L (2011). "Nicotine dependence and psychological distress: outcomes and clinical implications in smoking cessation". Psychology Research and Behavior Management. 4: 119–128. doi:10.2147/prbm.s14243. PMC 3218785. PMID 22114542.
- ^ Hollinger MA (19 October 2007). Introduction to Pharmacology (Third ed.). Abingdon: CRC Press. pp. 222–223. ISBN 978-1-4200-4742-4.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "The Medicines (Products Other Than Veterinary Drugs) (General Sale List) Amendment Order 2001". legislation.gov.uk. Retrieved 2 August 2022.
- ^ a b Nicotine. PubChem Compound Database. United States National Library of Medicine – National Center for Biotechnology Information. 16 February 2019. Retrieved 14 December 2024.
- ^ a b c d Landoni JH. "Nicotine (PIM)". INCHEM. International Programme on Chemical Safety. Retrieved 29 January 2019.
- ^ Fagerström K (December 2014). "Nicotine: Pharmacology, Toxicity and Therapeutic use". Journal of Smoking Cessation. 9 (2): 53–59. doi:10.1017/jsc.2014.27.
- ^ Sajja RK, Rahman S, Cucullo L (March 2016). "Drugs of abuse and blood-brain barrier endothelial dysfunction: A focus on the role of oxidative stress". Journal of Cerebral Blood Flow and Metabolism. 36 (3): 539–554. doi:10.1177/0271678X15616978. PMC 4794105. PMID 26661236.
- ^ "Nicotine: Clinical data". IUPHAR/BPS Guide to Pharmacology. International Union of Basic and Clinical Pharmacology.
Used as an aid to smoking cessation and for the relief of nicotine withdrawal symptoms.
- ^ Abou-Donia M (5 February 2015). Mammalian Toxicology. John Wiley & Sons. pp. 587–. ISBN 978-1-118-68285-2.
- ^ a b c d "Nicotinic acetylcholine receptors: Introduction". IUPHAR Database. International Union of Basic and Clinical Pharmacology. Archived from the original on 29 June 2017. Retrieved 1 September 2014.
- ^ a b Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 9: Autonomic Nervous System". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. p. 234. ISBN 978-0-07-148127-4.
Nicotine ... is a natural alkaloid of the tobacco plant. Lobeline is a natural alkaloid of Indian tobacco. Both drugs are agonists are nicotinic cholinergic receptors ...
- ^ Kishioka S, Kiguchi N, Kobayashi Y, Saika F (2014). "Nicotine effects and the endogenous opioid system". Journal of Pharmacological Sciences. 125 (2): 117–124. doi:10.1254/jphs.14R03CP. PMID 24882143.
- ^ "Smoking and Tobacco Control Monograph No. 9" (PDF). Archived (PDF) from the original on 9 October 2022. Retrieved 19 December 2012.
- ^ a b c d e f g h Siegmund B, Leitner E, Pfannhauser W (August 1999). "Determination of the nicotine content of various edible nightshades (Solanaceae) and their products and estimation of the associated dietary nicotine intake". Journal of Agricultural and Food Chemistry. 47 (8): 3113–20. Bibcode:1999JAFC...47.3113S. doi:10.1021/jf990089w. PMID 10552617.
- ^ Rodgman A, Perfetti TA (2009). The chemical components of tobacco and tobacco smoke. Boca Raton, FL: CRC Press. ISBN 978-1-4200-7883-1. LCCN 2008018913.[page needed]
- ^ a b c Ujváry I (1999). "Nicotine and Other Insecticidal Alkaloids". In Yamamoto I, Casida J (eds.). Nicotinoid Insecticides and the Nicotinic Acetylcholine Receptor. Tokyo: Springer-Verlag. pp. 29–69.
- ^ Perkins KA, Karelitz JL (August 2013). "Reinforcement enhancing effects of nicotine via smoking". Psychopharmacology. 228 (3): 479–486. doi:10.1007/s00213-013-3054-4. PMC 3707934. PMID 23494236.
- ^ a b Grana R, Benowitz N, Glantz SA (May 2014). "E-cigarettes: a scientific review". Circulation. 129 (19): 1972–1986. doi:10.1161/circulationaha.114.007667. PMC 4018182. PMID 24821826.
- ^ a b c Siqueira LM (January 2017). "Nicotine and Tobacco as Substances of Abuse in Children and Adolescents". Pediatrics. 139 (1): e20163436. doi:10.1542/peds.2016-3436. PMID 27994114.
- ^ Etter JF (July 2007). "Addiction to the nicotine gum in never smokers". BMC Public Health. 7: 159. doi:10.1186/1471-2458-7-159. PMC 1939993. PMID 17640334.
- ^ Olausson P, Jentsch JD, Taylor JR (January 2004). "Nicotine enhances responding with conditioned reinforcement". Psychopharmacology. 171 (2): 173–178. doi:10.1007/s00213-003-1575-y. PMID 13680077. S2CID 11855403.
- ^ a b "Evidence Review of E-Cigarettes and Heated Tobacco Products" (PDF). Public Health England. 2018.
- ^ "Tobacco more addictive than Nicotine". Archived from the original on 20 April 2023.
- ^ a b c d e Royal College of Physicians (28 April 2016). "Nicotine without smoke: Tobacco harm reduction". Retrieved 16 September 2020.
- ^ a b Smith TT, Rupprecht LE, Cwalina SN, Onimus MJ, Murphy SE, Donny EC, et al. (August 2016). "Effects of Monoamine Oxidase Inhibition on the Reinforcing Properties of Low-Dose Nicotine". Neuropsychopharmacology. 41 (9): 2335–2343. doi:10.1038/npp.2016.36. PMC 4946064. PMID 26955970.
- ^ a b c d Mayer B (January 2014). "How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century". Archives of Toxicology. 88 (1): 5–7. Bibcode:2014ArTox..88....5M. doi:10.1007/s00204-013-1127-0. PMC 3880486. PMID 24091634.
- ^ Caponnetto P, Campagna D, Papale G, Russo C, Polosa R (February 2012). "The emerging phenomenon of electronic cigarettes". Expert Review of Respiratory Medicine. 6 (1): 63–74. doi:10.1586/ers.11.92. PMID 22283580. S2CID 207223131.
- ^ Jain R, Mukherjee K, Balhara YP (April 2008). "The role of NMDA receptor antagonists in nicotine tolerance, sensitization, and physical dependence: a preclinical review". Yonsei Medical Journal. 49 (2): 175–188. doi:10.3349/ymj.2008.49.2.175. PMC 2615322. PMID 18452252.
- ^ Miyasato K (March 2013). "[Psychiatric and psychological features of nicotine dependence]". Nihon Rinsho. Japanese Journal of Clinical Medicine. 71 (3): 477–481. PMID 23631239.
- ^ a b c Parrott AC (July 2015). "Why all stimulant drugs are damaging to recreational users: an empirical overview and psychobiological explanation". Human Psychopharmacology. 30 (4): 213–24. doi:10.1002/hup.2468. PMID 26216554. S2CID 7408200.
- ^ Parrott AC (March 2006). "Nicotine psychobiology: how chronic-dose prospective studies can illuminate some of the theoretical issues from acute-dose research" (PDF). Psychopharmacology. 184 (3–4): 567–576. doi:10.1007/s00213-005-0294-y. PMID 16463194. S2CID 11356233.
- ^ a b c d e f g Parrott AC (April 2003). "Cigarette-derived nicotine is not a medicine". The World Journal of Biological Psychiatry. 4 (2): 49–55. doi:10.3109/15622970309167951. PMID 12692774. S2CID 26903942.
- ^ Schraufnagel DE, Blasi F, Drummond MB, Lam DC, Latif E, Rosen MJ, et al. (September 2014). "Electronic cigarettes. A position statement of the forum of international respiratory societies". American Journal of Respiratory and Critical Care Medicine. 190 (6): 611–618. doi:10.1164/rccm.201407-1198PP. PMID 25006874. S2CID 43763340.
- ^ "E-Cigarette Use Among Youth and Young Adults. 2016 Surgeon General's report.lts" (PDF). surgeongeneral.gov. Archived (PDF) from the original on 9 October 2022.
- ^ a b c Schraufnagel DE (March 2015). "Electronic Cigarettes: Vulnerability of Youth". Pediatric Allergy, Immunology, and Pulmonology. 28 (1): 2–6. doi:10.1089/ped.2015.0490. PMC 4359356. PMID 25830075.
- ^ IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Personal Habits and Indoor Combustions. Lyon (FR): International Agency for Research on Cancer; 2012. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 100E.) TOBACCO SMOKING.
- ^ a b "Does nicotine cause cancer?". European Code Against Cancer. World Health Organization – International Agency for Research on Cancer. Retrieved 23 January 2019.
- ^ a b c d e National Center for Chronic Disease Prevention Health Promotion (US) Office on Smoking Health (2014). The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, Chapter 5 - Nicotine. Surgeon General of the United States. pp. 107–138. PMID 24455788.
- ^ Kohlmeier KA (June 2015). "Nicotine during pregnancy: changes induced in neurotransmission, which could heighten proclivity to addict and induce maladaptive control of attention". Journal of Developmental Origins of Health and Disease. 6 (3): 169–181. doi:10.1017/S2040174414000531. PMID 25385318. S2CID 29298949.
- ^ a b c d e f g h i j "Nicotine". United States National Library of Medicine – Toxicology Data Network. Hazardous Substances Data Bank. 20 August 2009.
- ^ a b "Nicotine". European Chemicals Agency: Committee for Risk Assessment. September 2015. Retrieved 23 January 2019.
- ^ Effah F, Taiwo B, Baines D, Bailey A, Marczylo T (October 2022). "Pulmonary effects of e-liquid flavors: a systematic review". Journal of Toxicology and Environmental Health Part B: Critical Reviews. 25 (7): 343–371. Bibcode:2022JTEHB..25..343E. doi:10.1080/10937404.2022.2124563. PMC 9590402. PMID 36154615.
- ^ Lavoie FW, Harris TM (1991). "Fatal nicotine ingestion". The Journal of Emergency Medicine. 9 (3): 133–136. doi:10.1016/0736-4679(91)90318-a. PMID 2050970.
- ^ a b c d e f g h i j Hartmann-Boyce J, Chepkin SC, Ye W, Bullen C, Lancaster T (May 2018). "Nicotine replacement therapy versus control for smoking cessation". The Cochrane Database of Systematic Reviews. 5 (5): CD000146. doi:10.1002/14651858.CD000146.pub5. PMC 6353172. PMID 29852054.
There is high-quality evidence that all of the licensed forms of NRT (gum, transdermal patch, nasal spray, inhalator and sublingual tablets/lozenges) can help people who make a quit attempt to increase their chances of successfully stopping smoking. NRTs increase the rate of quitting by 50% to 60%, regardless of setting, and further research is very unlikely to change our confidence in the estimate of the effect. The relative effectiveness of NRT appears to be largely independent of the intensity of additional support provided to the individual.
A meta-analysis of adverse events associated with NRT included 92 RCTs and 28 observational studies, and addressed a possible excess of chest pains and heart palpitations among users of NRT compared with placebo groups (Mills 2010). The authors report an OR of 2.06 (95% CI 1.51 to 2.82) across 12 studies. We replicated this data collection exercise and analysis where data were available (included and excluded) in this review, and detected a similar but slightly lower estimate, OR 1.88 (95% CI 1.37 to 2.57; 15 studies; 11,074 participants; OR rather than RR calculated for comparison; Analysis 6.1). Chest pains and heart palpitations were an extremely rare event, occurring at a rate of 2.5% in the NRT groups compared with 1.4% in the control groups in the 15 trials in which they were reported at all. A recent network meta-analysis of cardiovascular events associated with smoking cessation pharmacotherapies (Mills 2014), including 21 RCTs comparing NRT with placebo, found statistically significant evidence that the rate of cardiovascular events with NRT was higher (RR 2.29 95% CI 1.39 to 3.82). However, when only serious adverse cardiac events (myocardial infarction, stroke and cardiovascular death) were considered, the finding was not statistically significant (RR 1.95 95% CI 0.26 to 4.30). - ^ Theodoulou A, Chepkin SC, Ye W, Fanshawe TR, Bullen C, Hartmann-Boyce J, et al. (June 2023). "Different doses, durations and modes of delivery of nicotine replacement therapy for smoking cessation". The Cochrane Database of Systematic Reviews. 2023 (6): CD013308. doi:10.1002/14651858.CD013308.pub2. PMC 10278922. PMID 37335995.
- ^ The MIND Study. "Why Nicotine?". MIND. Retrieved 6 December 2020.
- ^ Harris JG, Kongs S, Allensworth D, Martin L, Tregellas J, Sullivan B, et al. (July 2004). "Effects of nicotine on cognitive deficits in schizophrenia". Neuropsychopharmacology. 29 (7): 1378–1385. doi:10.1038/sj.npp.1300450. PMID 15138435.
- ^ a b Tomizawa M, Casida JE (2005). "Neonicotinoid insecticide toxicology: mechanisms of selective action". Annual Review of Pharmacology and Toxicology. 45: 247–68. doi:10.1146/annurev.pharmtox.45.120403.095930. PMID 15822177.
- ^ Rodgman A, Perfetti TA (2009). The chemical components of tobacco and tobacco smoke. Boca Raton, FL: CRC Press. ISBN 978-1-4200-7883-1. LCCN 2008018913.[page needed]
- ^ "Tobacco and its evil cousin nicotine are good as a pesticide – American Chemical Society". American Chemical Society. Retrieved 29 October 2018.
- ^ USEPA (3 June 2009). "Nicotine; Product Cancellation Order". Federal Register: 26695–26696. Retrieved 8 April 2012.
- ^ US Code of Federal Regulations. 7 CFR 205.602 – Nonsynthetic substances prohibited for use in organic crop production
- ^ Tharp C (5 September 2014). "Safety for Homemade Remedies for Pest Control" (PDF). Montana Pesticide Bulletin. Montana State University. Archived from the original (PDF) on 5 September 2014. Retrieved 21 September 2020.
- ^ a b Michalski B, Herrmann M, Solecki R (July 2017). "[How does a pesticide residue turn into a contaminant?]". Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz (in German). 60 (7): 768–773. doi:10.1007/s00103-017-2556-3. PMID 28508955. S2CID 22662492.
- ^ European Food Safety Authority (7 May 2009). "Potential risks for public health due to the presence of nicotine in wild mushrooms". EFSA Journal. 7 (5): 286r. doi:10.2903/j.efsa.2009.286r.
- ^ Abreu-Villaça Y, Levin ED (February 2017). "Developmental neurotoxicity of succeeding generations of insecticides". Environment International. 99: 55–77. Bibcode:2017EnInt..99...55A. doi:10.1016/j.envint.2016.11.019. PMC 5285268. PMID 27908457.
- ^ Valentine G, Sofuoglu M (May 2018). "Cognitive Effects of Nicotine: Recent Progress". Current Neuropharmacology. 16 (4). Bentham Science Publishers: 403–414. doi:10.2174/1570159X15666171103152136. PMC 6018192. PMID 29110618.
- ^ Heishman SJ, Kleykamp BA, Singleton EG (July 2010). "Meta-analysis of the acute effects of nicotine and smoking on human performance". Psychopharmacology. 210 (4): 453–69. doi:10.1007/s00213-010-1848-1. PMC 3151730. PMID 20414766.
- ^ Sarter M (August 2015). "Behavioral-Cognitive Targets for Cholinergic Enhancement". Current Opinion in Behavioral Sciences. 4: 22–26. doi:10.1016/j.cobeha.2015.01.004. PMC 5466806. PMID 28607947.
- ^ "Nicotine: Biological activity". IUPHAR/BPS Guide to Pharmacology. International Union of Basic and Clinical Pharmacology. Retrieved 7 February 2016.
Kis as follows; α2β4=9900nM [5], α3β2=14nM [1], α3β4=187nM [1], α4β2=1nM [4,6]. Due to the heterogeneity of nACh channels we have not tagged a primary drug target for nicotine, although the α4β2 is reported to be the predominant high affinity subtype in the brain which mediates nicotine addiction
- ^ Majdi A, Kamari F, Vafaee MS, Sadigh-Eteghad S (October 2017). "Revisiting nicotine's role in the ageing brain and cognitive impairment" (PDF). Reviews in the Neurosciences. 28 (7): 767–781. doi:10.1515/revneuro-2017-0008. PMID 28586306. S2CID 3758298.
- ^ Uban KA, Horton MK, Jacobus J, Heyser C, Thompson WK, Tapert SF, et al. (August 2018). "Biospecimens and the ABCD study: Rationale, methods of collection, measurement and early data". Developmental Cognitive Neuroscience. 32: 97–106. doi:10.1016/j.dcn.2018.03.005. PMC 6487488. PMID 29606560.
- ^ a b Stolerman IP, Jarvis MJ (January 1995). "The scientific case that nicotine is addictive". Psychopharmacology. 117 (1): 2–10, discussion 14–20. doi:10.1007/BF02245088. PMID 7724697. S2CID 8731555.
- ^ Wilder N, Daley C, Sugarman J, Partridge J (April 2016). "Nicotine without smoke: Tobacco harm reduction". UK: Royal College of Physicians. pp. 58, 125.
- ^ a b c El Sayed KA, Sylvester PW (June 2007). "Biocatalytic and semisynthetic studies of the anticancer tobacco cembranoids". Expert Opinion on Investigational Drugs. 16 (6): 877–87. doi:10.1517/13543784.16.6.877. PMID 17501699. S2CID 21302112.
- ^ Rahman MA, Hann N, Wilson A, Worrall-Carter L (2014). "Electronic cigarettes: patterns of use, health effects, use in smoking cessation and regulatory issues". Tobacco Induced Diseases. 12 (1): 21. doi:10.1186/1617-9625-12-21. PMC 4350653. PMID 25745382.
- ^ "2003: The 3rd Annual Year In Ideas; Nicotini, The". www.nytimes.com. The New York Times Magazine. Archived from the original on 27 May 2015. Retrieved 28 March 2024.
- ^ Little MA, Ebbert JO (2016). "The safety of treatments for tobacco use disorder". Expert Opinion on Drug Safety. 15 (3): 333–41. doi:10.1517/14740338.2016.1131817. PMID 26715118. S2CID 12064318.
- ^ Aubin HJ, Luquiens A, Berlin I (February 2014). "Pharmacotherapy for smoking cessation: pharmacological principles and clinical practice". British Journal of Clinical Pharmacology. 77 (2): 324–36. doi:10.1111/bcp.12116. PMC 4014023. PMID 23488726.
- ^ a b c Bailey SR, Crew EE, Riske EC, Ammerman S, Robinson TN, Killen JD (April 2012). "Efficacy and tolerability of pharmacotherapies to aid smoking cessation in adolescents". Paediatric Drugs. 14 (2): 91–108. doi:10.2165/11594370-000000000-00000. PMC 3319092. PMID 22248234.
- ^ "Electronic Cigarettes – What are the health effects of using e-cigarettes?" (PDF). Centers for Disease Control and Prevention. 22 February 2018. Archived (PDF) from the original on 9 October 2022.
Nicotine is a health danger for pregnant women and their developing babies.
- ^ Bruin JE, Gerstein HC, Holloway AC (August 2010). "Long-term consequences of fetal and neonatal nicotine exposure: a critical review". Toxicological Sciences. 116 (2): 364–74. doi:10.1093/toxsci/kfq103. PMC 2905398. PMID 20363831.
there is no safe dose of nicotine during pregnancy... The general consensus among clinicians is that more information is needed about the risks of NRT use during pregnancy before well-informed definitive recommendations can be made to pregnant women... Overall, the evidence provided in this review overwhelmingly indicates that nicotine should no longer be considered the safe component of cigarette smoke. In fact, many of the adverse postnatal health outcomes associated with maternal smoking during pregnancy may be attributable, at least in part, to nicotine alone.
- ^ Forest S (1 March 2010). "Controversy and evidence about nicotine replacement therapy in pregnancy". MCN: The American Journal of Maternal/Child Nursing. 35 (2): 89–95. doi:10.1097/NMC.0b013e3181cafba4. PMID 20215949. S2CID 27085986.
- ^ Barua RS, Rigotti NA, Benowitz NL, Cummings KM, Jazayeri MA, Morris PB, et al. (December 2018). "2018 ACC Expert Consensus Decision Pathway on Tobacco Cessation Treatment: A Report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents". Journal of the American College of Cardiology. 72 (25): 3332–3365. doi:10.1016/j.jacc.2018.10.027. PMID 30527452.
- ^ Sanner T, Grimsrud TK (2015). "Nicotine: Carcinogenicity and Effects on Response to Cancer Treatment - A Review". Frontiers in Oncology. 5: 196. doi:10.3389/fonc.2015.00196. PMC 4553893. PMID 26380225.
- ^ a b c d e f g h i j k l m n "Nicotine". Drugs.com. American Society of Health-System Pharmacists. Retrieved 24 January 2019.
- ^ Detailed reference list is located on a separate image page.
- ^ Vij K (2014). Textbook of Forensic Medicine & Toxicology: Principles & Practice (5th ed.). Elsevier Health Sciences. p. 525. ISBN 978-81-312-3623-9. Extract of page 525
- ^ "NICOTINE: Systemic Agent". 8 July 2021.
- ^ a b c "Nicotine", Dictionary of Toxicology, Singapore: Springer Nature, p. 691, 2024, doi:10.1007/978-981-99-9283-6_1860, ISBN 978-981-99-9282-9, retrieved 19 October 2024,
Nicotine is a colorless, water-soluble, and extremely hazardous alkaloid. It also has a terrible taste.
- ^ Royal College of Physicians. "Nicotine Without Smoke -- Tobacco Harm Reduction". p. 125. Retrieved 30 September 2020.
Use of nicotine alone, in the doses used by smokers, represents little if any hazard to the user.
- ^ Douglas CE, Henson R, Drope J, Wender RC (July 2018). "The American Cancer Society public health statement on eliminating combustible tobacco use in the United States". CA. 68 (4): 240–245. doi:10.3322/caac.21455. PMID 29889305. S2CID 47016482.
It is the smoke from combustible tobacco products—not nicotine—that injures and kills millions of smokers.
- ^ Dinakar C, O'Connor GT (October 2016). "The Health Effects of Electronic Cigarettes". The New England Journal of Medicine. 375 (14): 1372–1381. doi:10.1056/NEJMra1502466. PMID 27705269.
Beyond its addictive properties, short-term or long-term exposure to nicotine in adults has not been established as dangerous
- ^ a b Hartmann-Boyce J, Chepkin SC, Ye W, Bullen C, Lancaster T (May 2018). "Nicotine replacement therapy versus control for smoking cessation". The Cochrane Database of Systematic Reviews. 5 (5): CD000146. doi:10.1002/14651858.CD000146.pub5. PMC 6353172. PMID 29852054.
- ^ England LJ, Bunnell RE, Pechacek TF, Tong VT, McAfee TA (August 2015). "Nicotine and the Developing Human: A Neglected Element in the Electronic Cigarette Debate". American Journal of Preventive Medicine. 49 (2): 286–293. doi:10.1016/j.amepre.2015.01.015. PMC 4594223. PMID 25794473.
- ^ "Nicotine Transdermal Patch" (PDF). United States Food and Drug Administration. Retrieved 24 January 2019.
- ^ a b "Nicotrol NS" (PDF). United States Food and Drug Administration. Retrieved 24 January 2019.
- ^ "Nicotrol" (PDF). Pfizer. Retrieved 24 January 2019.
- ^ a b Garcia AN, Salloum IM (October 2015). "Polysomnographic sleep disturbances in nicotine, caffeine, alcohol, cocaine, opioid, and cannabis use: A focused review". The American Journal on Addictions. 24 (7): 590–598. doi:10.1111/ajad.12291. PMID 26346395. S2CID 22703103.
- ^ Boutrel B, Koob GF (September 2004). "What keeps us awake: the neuropharmacology of stimulants and wakefulness-promoting medications". Sleep. 27 (6): 1181–1194. doi:10.1093/sleep/27.6.1181. PMID 15532213.
- ^ Jaehne A, Loessl B, Bárkai Z, Riemann D, Hornyak M (October 2009). "Effects of nicotine on sleep during consumption, withdrawal and replacement therapy". Sleep Medicine Reviews (Review). 13 (5): 363–377. doi:10.1016/j.smrv.2008.12.003. PMID 19345124.
- ^ a b c d Benowitz NL, Burbank AD (August 2016). "Cardiovascular toxicity of nicotine: Implications for electronic cigarette use". Trends in Cardiovascular Medicine. 26 (6): 515–523. doi:10.1016/j.tcm.2016.03.001. PMC 4958544. PMID 27079891.
- ^ Nestler EJ, Barrot M, Self DW (September 2001). "DeltaFosB: a sustained molecular switch for addiction". Proceedings of the National Academy of Sciences of the United States of America. 98 (20): 11042–6. Bibcode:2001PNAS...9811042N. doi:10.1073/pnas.191352698. PMC 58680. PMID 11572966.
Although the ΔFosB signal is relatively long-lived, it is not permanent. ΔFosB degrades gradually and can no longer be detected in brain after 1–2 months of drug withdrawal ... Indeed, ΔFosB is the longest-lived adaptation known to occur in adult brain, not only in response to drugs of abuse, but to any other perturbation (that doesn't involve lesions) as well.
- ^ Nestler EJ (December 2012). "Transcriptional mechanisms of drug addiction". Clinical Psychopharmacology and Neuroscience. 10 (3): 136–143. doi:10.9758/cpn.2012.10.3.136. PMC 3569166. PMID 23430970.
The 35–37 kD ΔFosB isoforms accumulate with chronic drug exposure due to their extraordinarily long half-lives. ... As a result of its stability, the ΔFosB protein persists in neurons for at least several weeks after cessation of drug exposure. ... ΔFosB overexpression in nucleus accumbens induces NFκB
- ^ Dougherty J, Miller D, Todd G, Kostenbauder HB (December 1981). "Reinforcing and other behavioral effects of nicotine". Neuroscience and Biobehavioral Reviews. 5 (4): 487–495. doi:10.1016/0149-7634(81)90019-1. PMID 7322454. S2CID 10076758.
- ^ a b Belluzzi JD, Wang R, Leslie FM (April 2005). "Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats". Neuropsychopharmacology. 30 (4): 705–712. doi:10.1038/sj.npp.1300586. PMID 15496937.
- ^ "Acetaldehyde | RIVM".
- ^ Das S, Prochaska JJ (October 2017). "Innovative approaches to support smoking cessation for individuals with mental illness and co-occurring substance use disorders". Expert Review of Respiratory Medicine. 11 (10): 841–850. doi:10.1080/17476348.2017.1361823. PMC 5790168. PMID 28756728.
- ^ Heishman SJ, Kleykamp BA, Singleton EG (July 2010). "Meta-analysis of the acute effects of nicotine and smoking on human performance". Psychopharmacology. 210 (4): 453–69. doi:10.1007/s00213-010-1848-1. PMC 3151730. PMID 20414766.
The significant effects of nicotine on motor abilities, attention, and memory likely represent true performance enhancement because they are not confounded by withdrawal relief. The beneficial cognitive effects of nicotine have implications for initiation of smoking and maintenance of tobacco dependence.
- ^ Hughes JR (March 2007). "Effects of abstinence from tobacco: valid symptoms and time course". Nicotine & Tobacco Research. 9 (3): 315–327. doi:10.1080/14622200701188919. PMID 17365764.
- ^ Dugas EN, Sylvestre MP, O'Loughlin EK, Brunet J, Kakinami L, Constantin E, et al. (February 2017). "Nicotine dependence and sleep quality in young adults". Addictive Behaviors. 65: 154–160. doi:10.1016/j.addbeh.2016.10.020. PMID 27816041.
- ^ Cohrs S, Rodenbeck A, Riemann D, Szagun B, Jaehne A, Brinkmeyer J, et al. (May 2014). "Impaired sleep quality and sleep duration in smokers-results from the German Multicenter Study on Nicotine Dependence". Addiction Biology. 19 (3): 486–96. doi:10.1111/j.1369-1600.2012.00487.x. hdl:11858/00-001M-0000-0025-BD0C-B. PMID 22913370. S2CID 1066283.
- ^ Bruijnzeel AW (May 2012). "Tobacco addiction and the dysregulation of brain stress systems". Neuroscience and Biobehavioral Reviews. 36 (5): 1418–41. doi:10.1016/j.neubiorev.2012.02.015. PMC 3340450. PMID 22405889.
Discontinuation of smoking leads to negative affective symptoms such as depressed mood, increased anxiety, and impaired memory and attention...Smoking cessation leads to a relatively mild somatic withdrawal syndrome and a severe affective withdrawal syndrome that is characterized by a decrease in positive affect, an increase in negative affect, craving for tobacco, irritability, anxiety, difficulty concentrating, hyperphagia, restlessness, and a disruption of sleep. Smoking during the acute withdrawal phase reduces craving for cigarettes and returns cognitive abilities to pre-smoking cessation level
- ^ a b Nestler EJ (December 2013). "Cellular basis of memory for addiction". Dialogues in Clinical Neuroscience. 15 (4): 431–443. doi:10.31887/DCNS.2013.15.4/enestler. PMC 3898681. PMID 24459410.
- ^ a b Ruffle JK (November 2014). "Molecular neurobiology of addiction: what's all the (Δ)FosB about?". The American Journal of Drug and Alcohol Abuse. 40 (6): 428–37. doi:10.3109/00952990.2014.933840. PMID 25083822. S2CID 19157711.
The knowledge of ΔFosB induction in chronic drug exposure provides a novel method for the evaluation of substance addiction profiles (i.e. how addictive they are). Xiong et al. used this premise to evaluate the potential addictive profile of propofol (119). Propofol is a general anaesthetic, however its abuse for recreational purpose has been documented (120). Using control drugs implicated in both ΔFosB induction and addiction (ethanol and nicotine), ...
Conclusions
ΔFosB is an essential transcription factor implicated in the molecular and behavioral pathways of addiction following repeated drug exposure. The formation of ΔFosB in multiple brain regions, and the molecular pathway leading to the formation of AP-1 complexes is well understood. The establishment of a functional purpose for ΔFosB has allowed further determination as to some of the key aspects of its molecular cascades, involving effectors such as GluR2 (87,88), Cdk5 (93) and NFkB (100). Moreover, many of these molecular changes identified are now directly linked to the structural, physiological and behavioral changes observed following chronic drug exposure (60,95,97,102). New frontiers of research investigating the molecular roles of ΔFosB have been opened by epigenetic studies, and recent advances have illustrated the role of ΔFosB acting on DNA and histones, truly as a molecular switch (34). As a consequence of our improved understanding of ΔFosB in addiction, it is possible to evaluate the addictive potential of current medications (119), as well as use it as a biomarker for assessing the efficacy of therapeutic interventions (121,122,124). - ^ Marttila K, Raattamaa H, Ahtee L (July 2006). "Effects of chronic nicotine administration and its withdrawal on striatal FosB/DeltaFosB and c-Fos expression in rats and mice". Neuropharmacology. 51 (1): 44–51. doi:10.1016/j.neuropharm.2006.02.014. PMID 16631212. S2CID 8551216.
- ^ Tolentino J (7 May 2018). "The Promise of Vaping and the Rise of Juul". The New Yorker. Retrieved 29 June 2024.
- ^ Cardinale A, Nastrucci C, Cesario A, Russo P (January 2012). "Nicotine: specific role in angiogenesis, proliferation and apoptosis". Critical Reviews in Toxicology (Review). 42 (1): 68–89. doi:10.3109/10408444.2011.623150. PMID 22050423. S2CID 11372110.
- ^ National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Population Health and Public Health Practice Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems (2018). "Chapter 4: Nicotine". In Eaton DL, Kwan LY, Stratton K (eds.). Public Health Consequences of E-Cigarettes (Review). National Academies Press. ISBN 978-0-309-46834-3.
- ^ a b Schaal C, Chellappan SP (January 2014). "Nicotine-mediated cell proliferation and tumor progression in smoking-related cancers". Molecular Cancer Research (Review). 12 (1): 14–23. doi:10.1158/1541-7786.MCR-13-0541. PMC 3915512. PMID 24398389.
- ^ Merecz-Sadowska A, Sitarek P, Zielinska-Blizniewska H, Malinowska K, Zajdel K, Zakonnik L, et al. (January 2020). "A Summary of In Vitro and In Vivo Studies Evaluating the Impact of E-Cigarette Exposure on Living Organisms and the Environment". International Journal of Molecular Sciences (Review). 21 (2): 652. doi:10.3390/ijms21020652. PMC 7013895. PMID 31963832.
 This article incorporates text by Merecz-Sadowska A, Sitarek P, Zielinska-Blizniewska H, Malinowska K, Zajdel K, Zakonnik L, Zajdel R available under the CC BY 4.0 license.
This article incorporates text by Merecz-Sadowska A, Sitarek P, Zielinska-Blizniewska H, Malinowska K, Zajdel K, Zakonnik L, Zajdel R available under the CC BY 4.0 license.
- ^ Kothari AN, Mi Z, Zapf M, Kuo PC (2014). "Novel clinical therapeutics targeting the epithelial to mesenchymal transition". Clinical and Translational Medicine (Review). 3: 35. doi:10.1186/s40169-014-0035-0. PMC 4198571. PMID 25343018.
- ^ Knezevich A, Muzic J, Hatsukami DK, Hecht SS, Stepanov I (February 2013). "Nornicotine nitrosation in saliva and its relation to endogenous synthesis of N'-nitrosonornicotine in humans". Nicotine & Tobacco Research (Primary). 15 (2): 591–5. doi:10.1093/ntr/nts172. PMC 3611998. PMID 22923602.
- ^ "List of Classifications – IARC Monographs on the Identification of Carcinogenic Hazards to Humans". monographs.iarc.fr. Retrieved 22 July 2020.
- ^ Sanner T, Grimsrud TK (31 August 2015). "Nicotine: Carcinogenicity and Effects on Response to Cancer Treatment - A Review". Frontiers in Oncology (Review). 5: 196. doi:10.3389/fonc.2015.00196. PMC 4553893. PMID 26380225.
- ^ Ginzkey C, Steussloff G, Koehler C, Burghartz M, Scherzed A, Hackenberg S, et al. (August 2014). "Nicotine derived genotoxic effects in human primary parotid gland cells as assessed in vitro by comet assay, cytokinesis-block micronucleus test and chromosome aberrations test". Toxicology in Vitro. 28 (5): 838–846. Bibcode:2014ToxVi..28..838G. doi:10.1016/j.tiv.2014.03.012. PMID 24698733.
- ^ Ginzkey C, Friehs G, Koehler C, Hackenberg S, Hagen R, Kleinsasser NH (February 2013). "Assessment of nicotine-induced DNA damage in a genotoxicological test battery". Mutation Research. 751 (1): 34–39. Bibcode:2013MRGTE.751...34G. doi:10.1016/j.mrgentox.2012.11.004. PMID 23200805.
- ^ Ginzkey C, Stueber T, Friehs G, Koehler C, Hackenberg S, Richter E, et al. (January 2012). "Analysis of nicotine-induced DNA damage in cells of the human respiratory tract". Toxicology Letters. 208 (1): 23–29. doi:10.1016/j.toxlet.2011.09.029. PMID 22001448.
- ^ Behnke M, Smith VC (March 2013). "Prenatal substance abuse: short- and long-term effects on the exposed fetus". Pediatrics. 131 (3): e1009-24. doi:10.1542/peds.2012-3931. PMC 8194464. PMID 23439891.
- ^ "State Health Officer's Report on E-Cigarettes: A Community Health Threat" (PDF). California Department of Public Health. January 2015. Archived (PDF) from the original on 9 October 2022.
- ^ Holbrook BD (June 2016). "The effects of nicotine on human fetal development". Birth Defects Research. Part C, Embryo Today. 108 (2): 181–192. doi:10.1002/bdrc.21128. PMID 27297020.
- ^ Bruin JE, Gerstein HC, Holloway AC (August 2010). "Long-term consequences of fetal and neonatal nicotine exposure: a critical review". Toxicological Sciences. 116 (2): 364–374. doi:10.1093/toxsci/kfq103. PMC 2905398. PMID 20363831.
- ^ "Consumer Updates: Nicotine Replacement Therapy Labels May Change". FDA. 1 April 2013.
- ^ Toxicology and Applied Pharmacology. Vol. 44, Pg. 1, 1978.
- ^ a b Schep LJ, Slaughter RJ, Beasley DM (September 2009). "Nicotinic plant poisoning". Clinical Toxicology. 47 (8): 771–81. doi:10.1080/15563650903252186. PMID 19778187. S2CID 28312730.
- ^ Smolinske SC, Spoerke DG, Spiller SK, Wruk KM, Kulig K, Rumack BH (January 1988). "Cigarette and nicotine chewing gum toxicity in children". Human Toxicology. 7 (1): 27–31. doi:10.1177/096032718800700105. PMID 3346035. S2CID 27707333.
- ^ Furer V, Hersch M, Silvetzki N, Breuer GS, Zevin S (March 2011). "Nicotiana glauca (tree tobacco) intoxication--two cases in one family". Journal of Medical Toxicology. 7 (1): 47–51. doi:10.1007/s13181-010-0102-x. PMC 3614112. PMID 20652661.
- ^ Gehlbach SH, Williams WA, Perry LD, Woodall JS (September 1974). "Green-tobacco sickness. An illness of tobacco harvesters". JAMA. 229 (14): 1880–3. doi:10.1001/jama.1974.03230520022024. PMID 4479133.
- ^ "CDC – NIOSH Pocket Guide to Chemical Hazards – Nicotine". www.cdc.gov. Retrieved 20 November 2015.
- ^ Pomerleau OF, Pomerleau CS (1984). "Neuroregulators and the reinforcement of smoking: towards a biobehavioral explanation". Neuroscience and Biobehavioral Reviews. 8 (4): 503–13. doi:10.1016/0149-7634(84)90007-1. PMID 6151160. S2CID 23847303.
- ^ Pomerleau OF, Rosecrans J (1989). "Neuroregulatory effects of nicotine". Psychoneuroendocrinology. 14 (6): 407–23. doi:10.1016/0306-4530(89)90040-1. hdl:2027.42/28190. PMID 2560221. S2CID 12080532.
- ^ Katzung BG (2006). Basic and Clinical Pharmacology. New York: McGraw-Hill Medical. pp. 99–105.
- ^ Xiu X, Puskar NL, Shanata JA, Lester HA, Dougherty DA (March 2009). "Nicotine binding to brain receptors requires a strong cation-pi interaction". Nature. 458 (7237): 534–7. Bibcode:2009Natur.458..534X. doi:10.1038/nature07768. PMC 2755585. PMID 19252481.
- ^ Nesbitt P (1969). Smoking, physiological arousal, and emotional response. Unpublished doctoral dissertation, Columbia University.
- ^ Parrott AC (January 1998). "Nesbitt's Paradox resolved? Stress and arousal modulation during cigarette smoking". Addiction. 93 (1): 27–39. CiteSeerX 10.1.1.465.2496. doi:10.1046/j.1360-0443.1998.931274.x. PMID 9624709.
- ^ Wadgave U, Nagesh L (July 2016). "Nicotine Replacement Therapy: An Overview". International Journal of Health Sciences. 10 (3): 425–35. doi:10.12816/0048737. PMC 5003586. PMID 27610066.
- ^ Grizzell JA, Echeverria V (October 2015). "New Insights into the Mechanisms of Action of Cotinine and its Distinctive Effects from Nicotine". Neurochemical Research. 40 (10): 2032–46. doi:10.1007/s11064-014-1359-2. PMID 24970109. S2CID 9393548.
- ^ a b c Malenka RC, Nestler EJ, Hyman SE (2009). Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 369, 372–373. ISBN 978-0-07-148127-4.
- ^ a b Dickson SL, Egecioglu E, Landgren S, Skibicka KP, Engel JA, Jerlhag E (June 2011). "The role of the central ghrelin system in reward from food and chemical drugs" (PDF). Molecular and Cellular Endocrinology. 340 (1): 80–7. doi:10.1016/j.mce.2011.02.017. hdl:2077/26318. PMID 21354264. S2CID 206815322. Archived from the original (PDF) on 4 August 2020. Retrieved 23 September 2019.
This reward link comprises a dopamine projection from the ventral tegmental area (VTA) to the nucleus accumbens together with a cholinergic input, arising primarily from the laterodorsal tegmental area.
- ^ a b c Picciotto MR, Mineur YS (January 2014). "Molecules and circuits involved in nicotine addiction: The many faces of smoking". Neuropharmacology (Review). 76 (Pt B): 545–53. doi:10.1016/j.neuropharm.2013.04.028. PMC 3772953. PMID 23632083.
Rat studies have shown that nicotine administration can decrease food intake and body weight, with greater effects in female animals (Grunberg et al., 1987). A similar nicotine regimen also decreases body weight and fat mass in mice as a result of β4* nAChR-mediated activation of POMC neurons and subsequent activation of MC4 receptors on second order neurons in the paraventricular nucleus of the hypothalamus (Mineur et al., 2011).
- ^ Levine A, Huang Y, Drisaldi B, Griffin EA, Pollak DD, Xu S, et al. (November 2011). "Molecular mechanism for a gateway drug: epigenetic changes initiated by nicotine prime gene expression by cocaine". Science Translational Medicine. 3 (107): 107ra109. doi:10.1126/scitranslmed.3003062. PMC 4042673. PMID 22049069.
- ^ Volkow ND (November 2011). "Epigenetics of nicotine: another nail in the coughing". Science Translational Medicine. 3 (107): 107ps43. doi:10.1126/scitranslmed.3003278. PMC 3492949. PMID 22049068.
- ^ Yoshida T, Sakane N, Umekawa T, Kondo M (January 1994). "Effect of nicotine on sympathetic nervous system activity of mice subjected to immobilization stress". Physiology & Behavior. 55 (1): 53–7. doi:10.1016/0031-9384(94)90009-4. PMID 8140174. S2CID 37754794.
- ^ Marieb EN, Hoehn K (2007). Human Anatomy & Physiology (7th Ed.). Pearson. pp. ?. ISBN 978-0-8053-5909-1.[page needed]
- ^ Henningfield JE, Calvento E, Pogun S (2009). Nicotine Psychopharmacology. Handbook of Experimental Pharmacology. Vol. 192. Springer. pp. 35, 37. doi:10.1007/978-3-540-69248-5. ISBN 978-3-540-69248-5.
- ^ Le Houezec J (September 2003). "Role of nicotine pharmacokinetics in nicotine addiction and nicotine replacement therapy: a review". The International Journal of Tuberculosis and Lung Disease. 7 (9): 811–9. PMID 12971663.
- ^ Kolli AR, Calvino-Martin F, Kuczaj AK, Wong ET, Titz B, Xiang Y, et al. (January 2023). "Deconvolution of Systemic Pharmacokinetics Predicts Inhaled Aerosol Dosimetry of Nicotine". European Journal of Pharmaceutical Sciences. 180: 106321. doi:10.1016/j.ejps.2022.106321. PMID 36336278.
- ^ Benowitz NL, Jacob P, Jones RT, Rosenberg J (May 1982). "Interindividual variability in the metabolism and cardiovascular effects of nicotine in man". The Journal of Pharmacology and Experimental Therapeutics. 221 (2): 368–72. PMID 7077531.
- ^ Russell MA, Jarvis M, Iyer R, Feyerabend C (April 1980). "Relation of nicotine yield of cigarettes to blood nicotine concentrations in smokers". British Medical Journal. 280 (6219): 972–976. doi:10.1136/bmj.280.6219.972. PMC 1601132. PMID 7417765.
- ^ Bhalala O (Spring 2003). "Detection of Cotinine in Blood Plasma by HPLC MS/MS". MIT Undergraduate Research Journal. 8: 45–50. Archived from the original on 24 December 2013.
- ^ Hukkanen J, Jacob P, Benowitz NL (March 2005). "Metabolism and disposition kinetics of nicotine". Pharmacological Reviews. 57 (1): 79–115. doi:10.1124/pr.57.1.3. PMID 15734728. S2CID 14374018.
- ^ Petrick LM, Svidovsky A, Dubowski Y (January 2011). "Thirdhand smoke: heterogeneous oxidation of nicotine and secondary aerosol formation in the indoor environment". Environmental Science & Technology. 45 (1): 328–33. Bibcode:2011EnST...45..328P. doi:10.1021/es102060v. PMID 21141815. S2CID 206939025.
- ^ "The danger of third-hand smoke: Plain language summary – Petrick et al., "Thirdhand smoke: heterogeneous oxidation of nicotine and secondary aerosol formation in the indoor environment" in Environmental Science & Technology". The Column. Vol. 7, no. 3. Chromatography Online. 22 February 2011.
- ^ Benowitz NL, Herrera B, Jacob P (September 2004). "Mentholated cigarette smoking inhibits nicotine metabolism". The Journal of Pharmacology and Experimental Therapeutics. 310 (3): 1208–15. doi:10.1124/jpet.104.066902. PMID 15084646. S2CID 16044557.
- ^ "Nicotine' actions on energy balance: Friend or foe?". Pharmacology & Therapeutics. 219. March 2021.
- ^ a b c d Hu T, Yang Z, Li MD (December 2018). "Pharmacological Effects and Regulatory Mechanisms of Tobacco Smoking Effects on Food Intake and Weight Control". Journal of Neuroimmune Pharmacology. 13 (4): 453–466. doi:10.1007/s11481-018-9800-y. PMID 30054897. S2CID 51727199.
Nicotine's weight effects appear to result especially from the drug's stimulation of α3β4 nicotine acetylcholine receptors (nAChRs), which are located on pro-opiomelanocortin (POMC) neurons in the arcuate nucleus (ARC), leading to activation of the melanocortin circuit, which is associated with body weight. Further, α7- and α4β2-containing nAChRs have been implicated in weight control by nicotine.
- ^ "NFPA Hazard Rating Information for Common Chemicals". Archived from the original on 17 February 2015. Retrieved 15 March 2015.
- ^ a b c Metcalf RL (2007), "Insect Control", Ullmann's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, p. 9
- ^ "L-Nicotine Material Safety Data Sheet". Sciencelab.com, Inc.
- ^ a b c Henry TA (1949). The Plant Alkaloids (PDF) (4th ed.). Philadelphia, Toronto: The Blakiston Company. pp. 36–43.
- ^ Gause GF (1941). "Chapter V: Analysis of various biological processes by the study of the differential action of optical isomers". In Luyet BJ (ed.). Optical Activity and Living Matter. A series of monographs on general physiology. Vol. 2. Normandy, Missouri: Biodynamica.
- ^ a b Zhang H, Pang Y, Luo Y, Li X, Chen H, Han S, et al. (July 2018). "Enantiomeric composition of nicotine in tobacco leaf, cigarette, smokeless tobacco, and e-liquid by normal phase high-performance liquid chromatography". Chirality. 30 (7): 923–931. doi:10.1002/chir.22866. PMID 29722457.
- ^ Hellinghausen G, Lee JT, Weatherly CA, Lopez DA, Armstrong DW (June 2017). "Evaluation of nicotine in tobacco-free-nicotine commercial products". Drug Testing and Analysis. 9 (6): 944–948. doi:10.1002/dta.2145. PMID 27943582.
- ^ Jenssen BP, Boykan R (February 2019). "Electronic Cigarettes and Youth in the United States: A Call to Action (at the Local, National and Global Levels)". Children. 6 (2): 30. doi:10.3390/children6020030. PMC 6406299. PMID 30791645.
 This article incorporates text by Jenssen BP, Boykan R available under the CC BY 4.0 license.
This article incorporates text by Jenssen BP, Boykan R available under the CC BY 4.0 license.
- ^ a b c Pictet A, Rotschy A (1904). "Synthese des Nicotins" [Synthesis of nicotine]. Berichte der Deutschen Chemischen Gesellschaft (in German). 37 (2): 1225–1235. doi:10.1002/cber.19040370206.
- ^ Ho TL, Kuzakov EV (2004). "A New Approach to Nicotine: Symmetry Consideration for Synthesis Design". Helvetica Chimica Acta. 87 (10): 2712–2716. doi:10.1002/hlca.200490241.
- ^ Ye X, Zhang Y, Song X, Liu Q (2022). "Research Progress in the Pharmacological Effects and Synthesis of Nicotine". ChemistrySelect. 7 (12). doi:10.1002/slct.202104425. S2CID 247687372.
- ^ Lamberts BL, Dewey LJ, Byerrum RU (May 1959). "Ornithine as a precursor for the pyrrolidine ring of nicotine". Biochimica et Biophysica Acta. 33 (1): 22–6. doi:10.1016/0006-3002(59)90492-5. PMID 13651178.
- ^ Dawson RF, Christman DR, d'Adamo A, Solt ML, Wolf AP (1960). "The Biosynthesis of Nicotine from Isotopically Labeled Nicotinic Acids". Journal of the American Chemical Society. 82 (10): 2628–2633. Bibcode:1960JAChS..82.2628D. doi:10.1021/ja01495a059.
- ^ Ashihara H, Crozier A, Komamine A, eds. (7 June 2011). Plant metabolism and biotechnology. Cambridge: Wiley. ISBN 978-0-470-74703-2.[page needed]
- ^ Benowitz NL, Hukkanen J, Jacob P (1 January 2009). "Nicotine Chemistry, Metabolism, Kinetics and Biomarkers". Nicotine Psychopharmacology. Handbook of Experimental Pharmacology. Vol. 192. pp. 29–60. doi:10.1007/978-3-540-69248-5_2. ISBN 978-3-540-69246-1. PMC 2953858. PMID 19184645.
- ^ Baselt RC (2014). Disposition of Toxic Drugs and Chemicals in Man (10th ed.). Biomedical Publications. pp. 1452–6. ISBN 978-0-9626523-9-4.
- ^ Mündel T, Jones DA (July 2006). "Effect of transdermal nicotine administration on exercise endurance in men". Experimental Physiology. 91 (4): 705–13. doi:10.1113/expphysiol.2006.033373. PMID 16627574. S2CID 41954065.
- ^ Hellinghausen G, Roy D, Wang Y, Lee JT, Lopez DA, Weatherly CA, et al. (May 2018). "A comprehensive methodology for the chiral separation of 40 tobacco alkaloids and their carcinogenic E/Z-(R,S)-tobacco-specific nitrosamine metabolites". Talanta. 181: 132–141. doi:10.1016/j.talanta.2017.12.060. PMID 29426492.
- ^ Liu B, Chen Y, Ma X, Hu K (September 2019). "Site-specific peak intensity ratio (SPIR) from 1D 2H/1H NMR spectra for rapid distinction between natural and synthetic nicotine and detection of possible adulteration". Analytical and Bioanalytical Chemistry. 411 (24): 6427–6434. doi:10.1007/s00216-019-02023-6. PMID 31321470. S2CID 197593505.
- ^ Cheetham AG, Plunkett S, Campbell P, Hilldrup J, Coffa BG, Gilliland S, et al. (14 April 2022). Greenlief CM (ed.). "Analysis and differentiation of tobacco-derived and synthetic nicotine products: Addressing an urgent regulatory issue". PLOS ONE. 17 (4): e0267049. Bibcode:2022PLoSO..1767049C. doi:10.1371/journal.pone.0267049. PMC 9009602. PMID 35421170.
- ^ "Tobacco (leaf tobacco)". Transportation Information Service.
- ^ Baldwin IT (December 2001). "An ecologically motivated analysis of plant-herbivore interactions in native tobacco". Plant Physiology. 127 (4): 1449–1458. doi:10.1104/pp.010762. JSTOR 4280212. PMC 1540177. PMID 11743088.
- ^ N.d. Natural history-driven, plant-mediated RNAi-based study reveals CYP6B46's role in a nicotine-mediated antipredator herbivore defense | PNAS.
- ^ Kessler D, Bhattacharya S, Diezel C, Rothe E, Gase K, Schöttner M, et al. (August 2012). "Unpredictability of nectar nicotine promotes outcrossing by hummingbirds in Nicotiana attenuata". The Plant Journal. 71 (4): 529–538. doi:10.1111/j.1365-313X.2012.05008.x. PMID 22448647.
- ^ Domino EF, Hornbach E, Demana T (August 1993). "The nicotine content of common vegetables". The New England Journal of Medicine. 329 (6): 437. doi:10.1056/NEJM199308053290619. PMID 8326992.
- ^ Moldoveanu SC, Scott WA, Lawson DM (April 2016). "Nicotine Analysis in Several Non-Tobacco Plant Materials". Beiträge zur Tabakforschung International/Contributions to Tobacco Research. 27 (2): 54–59. doi:10.1515/cttr-2016-0008.
- ^ Henningfield JE, Zeller M (March 2006). "Nicotine psychopharmacology research contributions to United States and global tobacco regulation: a look back and a look forward". Psychopharmacology. 184 (3–4): 286–91. doi:10.1007/s00213-006-0308-4. PMID 16463054. S2CID 38290573.
- ^ Posselt W, Reimann L (1828). "Chemische Untersuchung des Tabaks und Darstellung eines eigenthümlich wirksamen Prinzips dieser Pflanze" [Chemical investigation of tobacco and preparation of a characteristically active constituent of this plant]. Magazin für Pharmacie (in German). 6 (24): 138–161.
- ^ Melsens LH (1843). "Note sur la nicotine" [Note on nicotine]. Annales de Chimie et de Physique. third series (in French). 9: 465–479, see especially page 470. [Note: The empirical formula that Melsens provides is incorrect because at that time, chemists used the wrong atomic mass for carbon (6 instead of 12).]
- ^ Pinner A, Wolffenstein R (1891). "Ueber Nicotin" [About nicotine]. Berichte der Deutschen Chemischen Gesellschaft (in German). 24: 1373–1377. doi:10.1002/cber.189102401242.
- ^ Pinner A (1893). "Ueber Nicotin. Die Constitution des Alkaloïds" [About nicotine: The Constitution of the Alkaloids]. Berichte der Deutschen Chemischen Gesellschaft (in German). 26: 292–305. doi:10.1002/cber.18930260165.
- ^ Pinner A (1893). "Ueber Nicotin. I. Mitteilung". Archiv der Pharmazie. 231 (5–6): 378–448. doi:10.1002/ardp.18932310508. S2CID 83703998.
- ^ Zhang S. "E-Cigs Are Going Tobacco-Free With Synthetic Nicotine". Wired. ISSN 1059-1028. Retrieved 11 October 2022.
- ^ a b Dale MM, Ritter JM, Fowler RJ, Rang HP. Rang & Dale's Pharmacology (6th ed.). Churchill Livingstone. p. 598. ISBN 978-0-8089-2354-1.
- ^ Connolly GN, Alpert HR, Wayne GF, Koh H (October 2007). "Trends in nicotine yield in smoke and its relationship with design characteristics among popular US cigarette brands, 1997-2005". Tobacco Control. 16 (5): e5. doi:10.1136/tc.2006.019695. PMC 2598548. PMID 17897974.
- ^ "Industry Documents Library". www.industrydocuments.ucsf.edu. Retrieved 11 October 2022.
- ^ Jewett C (8 March 2022). "The Loophole That's Fueling a Return to Teenage Vaping". The New York Times. ISSN 0362-4331. Retrieved 11 October 2022.
- ^ Jewett C (8 March 2022). "The Loophole That's Fueling a Return to Teenage Vaping". The New York Times. ISSN 0362-4331. Retrieved 11 October 2022.
- ^ Jordt SE (September 2021). "Synthetic nicotine has arrived". Tobacco Control. 32 (e1): tobaccocontrol–2021–056626. doi:10.1136/tobaccocontrol-2021-056626. PMC 8898991. PMID 34493630.
- ^ Center for Tobacco Products (26 September 2022). "Tobacco 21". FDA.
- ^ "21, 18, or 14: A look at the legal age for smoking around the world". Straits Times. 3 October 2017. Retrieved 1 March 2019.
- ^ a b Jacob M (1 March 1985). "Superman versus Nick O'Teen — a children's anti-smoking campaign". Health Education Journal. 44 (1): 15–18. doi:10.1177/001789698504400104. S2CID 71246970.
- ^ Becker R (26 April 2019). "Why Big Tobacco and Big Vape love comparing nicotine to caffeine". The Verge.
- ^ Mineur YS, Picciotto MR (December 2010). "Nicotine receptors and depression: revisiting and revising the cholinergic hypothesis". Trends in Pharmacological Sciences. 31 (12): 580–6. doi:10.1016/j.tips.2010.09.004. PMC 2991594. PMID 20965579.
- ^ Peters R, Poulter R, Warner J, Beckett N, Burch L, Bulpitt C (December 2008). "Smoking, dementia and cognitive decline in the elderly, a systematic review". BMC Geriatrics. 8: 36. doi:10.1186/1471-2318-8-36. PMC 2642819. PMID 19105840.
- ^ Henningfield JE, Zeller M (2009). "Nicotine Psychopharmacology: Policy and Regulatory". Nicotine Psychopharmacology. Handbook of Experimental Pharmacology. Vol. 192. pp. 511–34. doi:10.1007/978-3-540-69248-5_18. ISBN 978-3-540-69246-1. PMID 19184661.
- ^ Quik M, O'Leary K, Tanner CM (September 2008). "Nicotine and Parkinson's disease: implications for therapy". Movement Disorders. 23 (12): 1641–52. doi:10.1002/mds.21900. PMC 4430096. PMID 18683238.
- ^ a b Fujii T, Mashimo M, Moriwaki Y, Misawa H, Ono S, Horiguchi K, et al. (2017). "Expression and Function of the Cholinergic System in Immune Cells". Frontiers in Immunology. 8: 1085. doi:10.3389/fimmu.2017.01085. PMC 5592202. PMID 28932225.
- ^ Banala S, Arvin MC, Bannon NM, Jin XT, Macklin JJ, Wang Y, et al. (May 2018). "Photoactivatable drugs for nicotinic optopharmacology". Nature Methods. 15 (5): 347–350. doi:10.1038/nmeth.4637. PMC 5923430. PMID 29578537.
- ^ Holliday RS, Campbell J, Preshaw PM (July 2019). "Effect of nicotine on human gingival, periodontal ligament and oral epithelial cells. A systematic review of the literature". Journal of Dentistry. 86: 81–88. doi:10.1016/j.jdent.2019.05.030. PMID 31136818. S2CID 169035502.
- ^ Holliday R, Preshaw PM, Ryan V, Sniehotta FF, McDonald S, Bauld L, et al. (4 June 2019). "A feasibility study with embedded pilot randomised controlled trial and process evaluation of electronic cigarettes for smoking cessation in patients with periodontitis". Pilot and Feasibility Studies. 5 (1): 74. doi:10.1186/s40814-019-0451-4. PMC 6547559. PMID 31171977.
External links
[edit]- Alkaloids found in Erythroxylum coca
- Alkaloids found in Nicotiana
- Anorectics
- Drugs developed by Pfizer
- Drugs developed by GSK plc
- Anxiolytics
- Euphoriants
- Nicotinic agonists
- Nicotinic antagonists
- Plant toxin insecticides
- Pregnane X receptor agonists
- Pyridine alkaloids
- Pyrrolidine alkaloids
- Smoking
- Stimulants
- Teratogens
- 3-Pyridyl compounds




